Chemical equation facts for kids
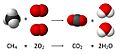
A chemical equation is like a special recipe for how chemicals react. It shows you what chemicals you start with and what new chemicals you get when they mix. Think of it like a math problem, but with chemical symbols instead of numbers!
Chemists use these equations to predict what will happen when different substances are combined. They can guess if a new chemical will form and what it will be.
Chemical equations can be written using words or special symbols for each chemical element. They also show how much of each chemical there is and its state:
- [s] for solid
- [l] for liquid
- [g] for gas
- [aq] for an aqueous solution (meaning it's dissolved in water)
For example, imagine mixing table salt dissolved in water with silver nitrate dissolved in water.
- Sodium chloride (NaCl[aq])
- Silver nitrate (AgNO3[aq])
When these two liquids mix, they form two new chemicals:
- Sodium nitrate (NaNO3[aq])
- Silver chloride (AgCl[s])
In symbols, this reaction looks like this: NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
Notice that the silver chloride (AgCl) is a solid. When a solid forms from two liquids and doesn't dissolve, it's called a precipitate. The whole reaction is known as a precipitation reaction.
Contents
Why We Balance Chemical Equations
Chemical equations must be balanced to follow an important rule called the law of conservation of mass. This law says that in a closed system, matter can't be created or destroyed. It just changes form!
So, in a chemical reaction, the total number of atoms of each element you start with (on the "reactant" side) must be the same as the total number of atoms of each element you end up with (on the "product" side).
Think of it like building with LEGOs. If you start with 10 red bricks and 5 blue bricks, you must still have 10 red bricks and 5 blue bricks in your final creation, even if they're arranged differently.
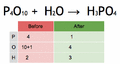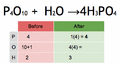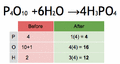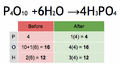
Balancing an equation means making sure the atoms on both sides are equal. We do this by adding numbers called coefficients in front of the chemical formulas. You should never change the small numbers (subscripts) within a chemical formula, because that would change the substance itself!
To find the number of atoms of an element, you multiply the coefficient by the subscript in its formula.
How to Balance Chemical Equations
Balancing chemical equations is often done by trial and error. It's like solving a puzzle!
The most common way to balance an equation is called the inspection method. This method works best for simpler reactions. You look at the equation and adjust the coefficients until the number of atoms for each element is the same on both sides. It takes practice, but you'll get better at it!
Let's look at an example: H2 + O2 → H2O
1. Hydrogen (H): There are 2 H atoms on the left (H2) and 2 H atoms on the right (H2O). Hydrogen is balanced! 2. Oxygen (O): There are 2 O atoms on the left (O2) but only 1 O atom on the right (H2O). Oxygen is not balanced.
To balance oxygen, we need 2 O atoms on the right. We can put a coefficient of 2 in front of H2O: H2 + O2 → 2H2O
Now let's check again: 1. Hydrogen (H): There are 2 H atoms on the left. On the right, 2H2O means 2 x 2 = 4 H atoms. Hydrogen is now unbalanced! 2. Oxygen (O): There are 2 O atoms on the left. On the right, 2H2O means 2 x 1 = 2 O atoms. Oxygen is balanced!
Since hydrogen is now unbalanced, we need to go back and fix it. We need 4 H atoms on the left. We can put a coefficient of 2 in front of H2: 2H2 + O2 → 2H2O
Let's check one last time: 1. Hydrogen (H): On the left, 2H2 means 2 x 2 = 4 H atoms. On the right, 2H2O means 2 x 2 = 4 H atoms. Hydrogen is balanced! 2. Oxygen (O): On the left, O2 means 2 O atoms. On the right, 2H2O means 2 x 1 = 2 O atoms. Oxygen is balanced!
Now the equation is balanced!
Images for kids
See also
 In Spanish: Ecuación química para niños
In Spanish: Ecuación química para niños