Aqueous solution facts for kids
An aqueous solution is a special kind of solution where water is the main liquid that does the dissolving. In chemistry, water is often called the "universal solvent" because it can dissolve so many different things. When something dissolves in water, it forms an aqueous solution.
Contents
What is an Aqueous Solution?
When you mix a substance with water and it seems to disappear, it has probably dissolved. The mixture you get is called a solution. If water is the liquid that does the dissolving, then it's an aqueous solution. For example, when you stir table salt into water, the salt dissolves, creating an aqueous solution of salt water.
Solvents and Solutes
In any solution, there are two main parts:
- The solvent is the liquid that does the dissolving. In an aqueous solution, the solvent is always water.
- The solute is the substance that gets dissolved. This can be a solid, a liquid, or even a gas. For instance, in salt water, salt is the solute.
How Things Dissolve in Water
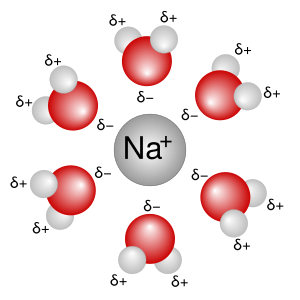
Water molecules have a special shape that makes them very good at dissolving many substances. They are like tiny magnets with a positive and negative end. When a substance like salt (which is made of charged particles called ions) is put into water, the water molecules surround these particles. They pull them apart and spread them out evenly in the water. This process is called solvation.
Common Examples of Aqueous Solutions
Aqueous solutions are all around us and very important in daily life:
- Salt water: This is a simple example where salt dissolves in water.
- Sugar water: Sugar dissolves in water to make a sweet drink.
- Soft drinks: These are mostly water with dissolved sugar, flavors, and carbon dioxide gas.
- Body fluids: Your blood, tears, and other body fluids are mostly aqueous solutions. They carry important nutrients and chemicals throughout your body.
- Cleaning products: Many household cleaners are aqueous solutions designed to dissolve dirt and grease.
Writing Aqueous Solutions in Chemistry
In chemical equations, chemists use a special symbol to show that a substance is dissolved in water. They add `(aq)` right after the formula of the substance.
- For example, if you dissolve table salt (which has the chemical formula NaCl) in water, you would write it as `NaCl(aq)`.
- This tells you that the salt is not a solid, but rather dissolved in water.
- Another example is sugar, which is `C₁₂H₂₂O₁₁`. When dissolved in water, it becomes `C₁₂H₂₂O₁₁(aq)`.
This `(aq)` notation is a quick way for scientists to understand the state of chemicals in a reaction. It shows that water is involved as the solvent.
See also
 In Spanish: Disolución acuosa para niños
In Spanish: Disolución acuosa para niños