Oxygen facts for kids

Liquid oxygen boiling
|
||||||||||||||||||||||||
| Oxygen | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | O2, O3 (ozone) and more (see Allotropes of oxygen) | |||||||||||||||||||||||
| Appearance | colorless gas; light blue liquid. | |||||||||||||||||||||||
| Standard atomic weight Ar, std(O) | [15.99903, 15.99977] conventional: 15.999 | |||||||||||||||||||||||
| Oxygen in the periodic table | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Atomic number (Z) | 8 | |||||||||||||||||||||||
| Group | group 16 (chalcogens) | |||||||||||||||||||||||
| Period | period 2 | |||||||||||||||||||||||
| Block | p | |||||||||||||||||||||||
| Electron configuration | [He] 2s2 2p4 | |||||||||||||||||||||||
| Electrons per shell | 2, 6 | |||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||
| Phase at STP | gas | |||||||||||||||||||||||
| Melting point | 54.36 K (-218.79 °C, -361.82 °F) | |||||||||||||||||||||||
| Boiling point | 90.20 K (-182.95 °C, -297.31 °F) | |||||||||||||||||||||||
| Density (at STP) | 1.429 g/L | |||||||||||||||||||||||
| when liquid (at b.p.) | 1.141 g/cm3 | |||||||||||||||||||||||
| Critical point | 154.59 K, 5.043 MPa | |||||||||||||||||||||||
| Heat of fusion | (O2) 0.444 kJ/mol | |||||||||||||||||||||||
| Heat of vaporization | (O2) 6.82 kJ/mol | |||||||||||||||||||||||
| Molar heat capacity | (O2) 29.378 J/(mol·K) |
|||||||||||||||||||||||
Vapor pressure
|
||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||
| Oxidation states | −1, −2, +1, +2 | |||||||||||||||||||||||
| Electronegativity | Pauling scale: 3.44 | |||||||||||||||||||||||
| Ionization energies |
|
|||||||||||||||||||||||
| Covalent radius | 66±2 pm | |||||||||||||||||||||||
| Van der Waals radius | 152 pm | |||||||||||||||||||||||
| Spectral lines of oxygen | ||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||
| Crystal structure | cubic | |||||||||||||||||||||||
| Speed of sound | (gas, 27 °C) 330 m/s | |||||||||||||||||||||||
| Thermal conductivity | 26.58x10-3 W/(m⋅K) | |||||||||||||||||||||||
| Magnetic ordering | paramagnetic | |||||||||||||||||||||||
| CAS Number | 7782-44-7 | |||||||||||||||||||||||
| History | ||||||||||||||||||||||||
| Discovery | Carl Wilhelm Scheele (1772) | |||||||||||||||||||||||
| Named by | Antoine Lavoisier (1777) | |||||||||||||||||||||||
| Main isotopes of oxygen | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
Oxygen is a chemical element with the symbol O. It is the third most common element in the universe. Only hydrogen and helium are more common. When alone, two oxygen atoms usually join together. They form dioxygen (O2), which is a colourless gas. It has no taste or smell.
As a liquid or solid, oxygen is a pale blue color. Dioxygen gas makes up about 20.8% of the Earth's atmosphere. Oxygen is part of the chalcogen group on the periodic table. Its atomic number is 8. It is a very reactive nonmetal. It also forms oxides with many other elements. Oxides make up almost half of the Earth's crust.
Most life on Earth uses oxygen gas (O2) for respiration. Many organic molecules in living things contain oxygen. These include proteins, nucleic acids, carbohydrates, and fats. Oxygen is also a part of water, which all known life needs to survive. Plants create the Earth's dioxygen through photosynthesis. They use the Sun's light to separate oxygen from water and carbon dioxide. Ozone (O3) is found high in the Earth's atmosphere. This ozone layer absorbs harmful ultraviolet radiation. This helps protect life on the ground.
Oxygen was first isolated by Michael Sendivogius before 1604. However, its discovery is often linked to Carl Wilhelm Scheele in Sweden (1773). It is also linked to Joseph Priestley in England (1774). Priestley is usually seen as the main discoverer. This is because his work was published first. He called it "dephlogisticated air" and did not realize it was a chemical element. Antoine Lavoisier gave the element the name oxygen in 1777. He was the first to correctly explain how it helps combustion (burning).
Oxygen is used for making steel, plastics, and textiles. It is also used in rocket propellant and for welding.
Contents
Oxygen's Story: How We Found It
Early Science Experiments
One of the first known experiments on how combustion (burning) needs air was done by Greek Philo of Byzantium. This was in the 2nd century BC. He wrote that if you turn a vessel upside down over a burning candle, water would rise into it. Philo wrongly thought the air turned into fire. Much later, Leonardo da Vinci correctly figured out that air was used up during burning. This caused the water to rise.
In the late 1600s, Robery Boyle found that air is needed for burning. English chemist John Mayow added to this idea. He showed that fire only needed a part of the air. We now call this part oxygen. In one experiment, he put a candle in a closed container. Water rose to replace one-fourteenth of the air's volume before the candle went out. The same thing happened when a mouse was put into the box. From this, he realized that oxygen is used for both respiration (breathing) and burning.
The Phlogiston Theory
Scientists like Robert Hooke, Ole Borch, Mikhail Lomonosov, and Pierre Bayen all made oxygen in experiments. This was in the 17th and 18th centuries. But none of them thought it was a chemical element. This was likely because of the phlogiston theory. Most people at the time believed this theory explained burning and corrosion (like rusting).
J. J. Becher came up with this theory in 1667. Georg Ernst Stahl added to it in 1731. The phlogiston theory said that all things that could burn were made of two parts. One part, called phlogiston, was released when the material burned.
Materials that burned easily, like wood or coal, were thought to have a lot of phlogiston. Things that corroded, like iron, were thought to have only a little. Air was not part of this theory.
Discovering Oxygen
Polish alchemist and physician Michael Sendivogius wrote about a substance in air. He called it the "food of life." This substance is oxygen. Between 1598 and 1604, Sendivogius found that this substance was the same as what was made when potassium nitrate was heated. Some people believe this was the first discovery of oxygen.
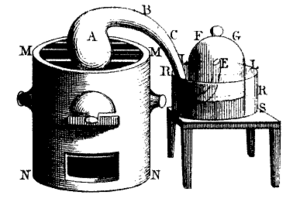
It is also often said that oxygen was first found by Swedish pharmacist Carl Wilhelm Scheele. He made oxygen by heating mercuric oxide and some nitrates in 1771. Scheele called the gas "fire air." This was because it was the only gas known to allow burning. He published his discovery in 1777.
On August 1, 1774, British clergyman Joseph Priestley did an experiment. He focused sunlight on mercuric oxide in a glass tube. This created a gas he called "dephlogisticated air." He also found that candles burned brighter in this gas. And mice lived longer while breathing it. When he breathed the gas himself, he said it felt like normal air. But his lungs felt lighter and easy afterward. His findings were published in 1775. Because his work was published first, he is usually credited with discovering oxygen.
French chemist Antoine Lavoisier later claimed he had discovered the substance too. Priestley visited him in 1774 and told him about his experiment. Scheele also sent a letter to Lavoisier that year about his discovery.
Lavoisier's Important Work
Lavoisier performed the first major experiments on oxidation. He also gave the first correct explanation of how burning works. He used these experiments to show that the phlogiston theory was wrong. He also tried to prove that the substance Priestley and Scheele found was a true chemical element.
In one experiment, Lavoisier found that the mass did not increase when tin and air were heated in a closed container. He also noticed that air rushed in when the container was opened. After this, he found that the tin had gained the same amount of mass as the air that rushed in. He published his findings in 1777. He wrote that air was made of two gases. One he called "vital air" (oxygen), which is needed for burning and breathing. The other he called "azote" (nitrogen), which means "lifeless" in Greek language. This is still the name for nitrogen in some languages, like French.
Lavoisier renamed "vital air" to "oxygène." This means "producer from acids" in Greek. He called it this because he thought oxygen was in all acids. This idea was later found to be wrong. Many chemists realized Lavoisier's naming was incorrect. However, the name was already too common to change. "Oxygen" became the name in the English language, even though some English scientists were against it.
Later Discoveries
John Dalton's theory of atoms said that all elements had one atom. He also thought atoms in compounds were usually alone. For example, he wrongly believed that water (H2O) had the formula HO. In 1805, Joseph Louis Gay-Lussac and Alexander von Humboldt showed that water is made of two hydrogen atoms and one oxygen atom. By 1811, Amedeo Avogadro correctly figured out what water was made of, based on Avogadro's law.
By the late 1800s, scientists found that air could be turned into a liquid. The parts of air could then be separated by compressing and cooling it. Swiss chemist and physicist Raoul Pictet discovered liquid oxygen. He did this by evaporating sulfur dioxide to turn carbon dioxide into a liquid. This liquid was then evaporated to cool oxygen gas, turning it into a liquid. He sent a telegram to the French Academy of Sciences on December 22, 1877, announcing his discovery.
What Makes Oxygen Special?
Properties and Structure
At normal temperature and pressure, oxygen is a gas. It has no colour, odour, or taste. Its chemical formula is O2, and it is called dioxygen.
In dioxygen, two oxygen atoms are chemically bound together. This bond is a covalent double bond. Dioxygen is very reactive. It can react with many other elements. When metal elements react with dioxygen, oxides are formed. An example is iron oxide, which we know as rust. There are many oxide compounds found on Earth.
Different Forms of Oxygen (Allotropes)
The most common form, or allotrope, of oxygen on Earth is dioxygen (O2). This is the second largest part of the Earth's atmosphere, after dinitrogen (N2). O2 has a bond length of 121 pm. Its bond energy is 498 kJ/mol. Because of its energy, O2 is used by complex life forms like animals.
Ozone (O3) is another form of oxygen. It is very reactive and can harm the lungs if breathed in. Ozone forms in the upper atmosphere. This happens when O2 combines with pure oxygen. The pure oxygen is made when O2 is split by ultraviolet radiation. Ozone absorbs a lot of UV radiation. So, the ozone layer in the upper atmosphere protects Earth from this radiation.
Tetraoxygen (O4) was found in 2001. It only exists under extreme conditions, when a lot of pressure is put on O2.
Physical Characteristics
Oxygen dissolves more easily from air into water than nitrogen does. In air, there is about one oxygen molecule for every four nitrogen molecules (a ratio of 1:4). But in water, there is one O2 molecule for every two N2 molecules (a 1:2 ratio). It is also easier for O2 to dissolve in freshwater than in seawater.
Oxygen condenses into a liquid at 90.20 K (-182.95°C, -297.31 °F). It freezes into a solid at 54.36 K (-218.79 °C, -361.82 °F). Both liquid and solid O2 are clear with a light-blue color. Oxygen is very reactive. It must be kept away from anything that can burn.
Oxygen's Isotopes
There are three stable isotopes of oxygen found in nature. They are 16O, 17O, and 18O. About 99.7% of all oxygen is the 16O isotope.
Where is Oxygen Found?
| Z | Element | Mass fraction in parts per million | ||
|---|---|---|---|---|
| 1 | Hydrogen | 739,000 | 71 × mass of oxygen (red bar) | |
| 2 | Helium | 240,000 | 23 × mass of oxygen (red bar) | |
| 8 | Oxygen | 10,400 |
|
|
| 6 | Carbon | 4,600 |
|
|
| 10 | Neon | 1,340 |
|
|
Oxygen is the most common element by mass on Earth. It is the third most common element in the universe. Only hydrogen and helium are more common. About 0.9% of the Sun's mass is oxygen. Oxygen makes up 49.2% of the Earth's crust by mass. It is found as part of oxide compounds like silicon dioxide. It is also the main part of the Earth's oceans, making up 88.8% by mass.
Oxygen gas is the second most common part of the atmosphere. It makes up 20.8% of its mass and 23.1% of its volume. Earth is unusual compared to other known planets. A large amount of its atmosphere is oxygen gas. Mars has only 0.1% O2 by volume. Other planets in the Solar System have even less.
The high amount of oxygen gas on Earth is due to the oxygen cycle. This cycle is mainly controlled by photosynthesis. Photosynthesis creates oxygen gas from carbon dioxide, water, and the Sun's energy. Respiration then takes oxygen gas out of the atmosphere. It turns it back into carbon dioxide and water. These two processes happen at the same rate. So, the amount of oxygen gas and carbon dioxide stays balanced.
How We Use Oxygen
Oxygen in Medicine
O2 is a very important part of respiration (breathing). Because of this, it is used in medicine. It helps increase the amount of oxygen in a person's blood. This allows more respiration to happen. It can help people get healthy faster if they are sick. Oxygen therapy is used to treat conditions like emphysema, pneumonia, and some heart problems. It also helps with any disease that makes it hard for a person to take in oxygen.
Oxygen for Life Support
Low-pressure O2 is used in space suits. It surrounds the body with the gas. Pure oxygen is used, but at a much lower pressure than on Earth. If the pressure were higher, it could be harmful.
Staying Safe with Oxygen
| O2 |
Oxygen's NFPA 704 rating (shown on the right) says that compressed oxygen gas is not dangerous to health and is not flammable on its own.
Oxygen Toxicity
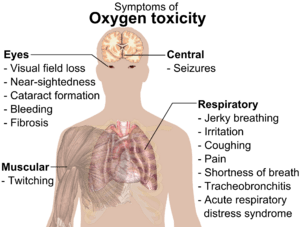
At high pressures, oxygen gas (O2) can be dangerous to animals, including humans. It can cause convulsions and other health problems. Oxygen toxicity usually starts to happen at pressures more than 50 kilopascals (kPa). This is like breathing air with about 50% oxygen at normal pressure. (Air on Earth has around 20% oxygen).
In the past, premature babies were sometimes placed in boxes with air that had a lot of O2. This practice was stopped when some babies went blind because of the high oxygen levels. Breathing pure O2 in space suits does not cause harm because a much lower pressure is used.
Fire and Other Dangers
High amounts of pure O2 can cause a very fast fire. When concentrated oxygen and fuels are brought close together, even a small ignition can cause a huge fire. The Apollo 1 crew tragically lost their lives in a fire. This happened because of concentrated oxygen used in the air of their capsule.
If liquid oxygen spills onto organic compounds, like wood, it can explode.
Images for kids
-
Joseph Priestley is often given credit for the discovery.
-
Antoine Lavoisier showed that the phlogiston theory was wrong.
-
Robert H. Goddard and a liquid oxygen-gasoline rocket.
-
Low pressure pure O2 is used in space suits.
-
Most commercial O2 is used to process iron.
-
Water (H2O) is the most common oxygen compound.
-
Oxides, like iron oxide or rust, form when oxygen combines with other elements.
-
The inside of the Apollo 1 Command Module after the fire. Pure O2 at high pressure and a spark led to the fire.
See also
 In Spanish: Oxígeno para niños
In Spanish: Oxígeno para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |