Nitrate facts for kids
Quick facts for kids Nitrate |
|
|---|---|
 |
|
|
Nitrate
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
| Infobox references | |
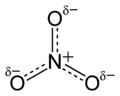
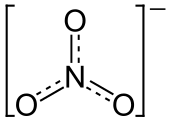
Nitrate is a special group of atoms called an ion. An ion is an atom or group of atoms that has an electric charge. Nitrate has a negative charge. Its chemical formula is NO3-. This means it has one nitrogen atom and three oxygen atoms.
Nitrate is a very important part of many different molecules. You can find it in things like fertilizers and even some foods.
Contents
What is Nitrate?
Nitrate is known as a polyatomic ion. This means it is made of more than one atom. In the case of nitrate, it has one nitrogen atom and three oxygen atoms. These atoms are held together by strong chemical bonds. The whole group has a charge of negative one. This charge makes it react with other atoms and molecules.
How Nitrates are Used
Nitrates are used in many ways in our daily lives.
Helping Plants Grow
One of the most common uses for nitrates is in fertilizers. Plants need nitrogen to grow big and strong. Nitrates provide this important nutrient. For example, Potassium nitrate is a common type of nitrate used in fertilizers. It gives plants both potassium and nitrogen.
Preserving Food
Nitrates also help keep food fresh for longer. Sodium nitrate is often used to preserve meats. It helps stop bacteria from growing. This keeps the food safe to eat and tasting good.
Other Uses
Some types of nitrates can be explosive. They are used in certain industrial processes. Large amounts of nitrates are made from a chemical called ammonia.
Nitrate in Nature
Nitrates are a key part of the nitrogen cycle on Earth. This cycle describes how nitrogen moves through the air, soil, and living things. Plants take up nitrates from the soil. Animals then get nitrogen by eating plants. When plants and animals die, nitrogen returns to the soil.
Nitrate, Nitrite, and Nitric Acid
It can be easy to confuse nitrate with similar chemicals.
Nitrate vs. Nitrite
Nitrates are similar to nitrites. The main difference is the number of oxygen atoms. Nitrate has three oxygen atoms (NO3-). Nitrite has two oxygen atoms (NO2-). Both are important in chemistry and biology.
Nitrate vs. Nitric Acid
Nitric acid has the formula HNO3. It is different from nitrate because it also includes a hydrogen atom. Nitric acid does not have an overall charge. This is because the hydrogen ion has a positive charge, which balances the negative charge of the nitrate part.
Making Nitrates
Many nitrates are made by humans. They are often produced from ammonia. Ammonia is a compound of nitrogen and hydrogen. This process allows us to create the large amounts of nitrates needed for fertilizers and other uses.
Images for kids
See also
 In Spanish: Nitrato para niños
In Spanish: Nitrato para niños
 | James B. Knighten |
 | Azellia White |
 | Willa Brown |