Nitrogen cycle facts for kids
The nitrogen cycle is a super important process that changes nitrogen into different forms. These forms can then be used by all living things, like plants, animals, and even tiny microbes!
Did you know that the air we breathe is mostly nitrogen? About 78% of the air is nitrogen gas (N2). Nitrogen is a key ingredient for life. It's a big part of things like proteins, DNA, and RNA, which are the building blocks of every living cell. Plants also need nitrogen to grow and make their own food through photosynthesis.
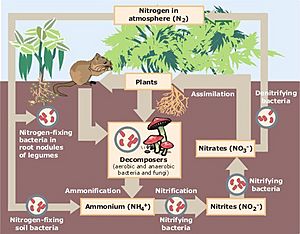
But here's the tricky part: most living things can't use the nitrogen gas directly from the air. It needs to be changed first! This special process is called nitrogen fixation.
Contents
Nitrogen Fixation: Changing Air into Food
Most nitrogen fixation is done by tiny living things called bacteria. These amazing bacteria have a special tool (an enzyme) that helps them combine nitrogen gas (N2) from the air with hydrogen gas (H2). When they do this, they create ammonia (NH3). Ammonia is a form of nitrogen that plants can actually use!
Some of these helpful bacteria live right inside the roots of certain plants, especially legumes like beans and peas. It's a teamwork effort! The bacteria make ammonia for the plant, and in return, the plant gives the bacteria sugary foods called carbohydrates. Other plants get their nitrogen from different nitrogen compounds that are already in the soil. They simply soak it up through their roots. And how do animals get nitrogen? We get it by eating plants or by eating other animals that have eaten plants!
Ammonification: Nitrogen from Decomposers
When plants and animals die, or when animals produce waste, the nitrogen in their bodies doesn't just disappear. Instead, other tiny living things called decomposers, which include certain bacteria and fungi, get to work. They break down the dead stuff into its basic parts. This process is called ammonification.
During ammonification, these decomposers create ammonium (NH4) in the soil. Ammonium has a positive electrical charge, which means it can easily stick to parts of the soil like clay and humus.
Nitrification: Making Nitrogen Usable and Movable
Ammonia and ammonium can be harmful to fish and other water animals if there's too much of it. That's why people often check the nitrogen levels in sewage and other waste-water. If ammonia levels get too high, another important process called nitrification needs to happen.
Nitrification is when special bacteria change ammonia and ammonium into other forms of nitrogen: first into nitrite (NO2−) and then into nitrate (NO3−).
Unlike ammonium, nitrite and nitrate have a negative electrical charge. This means they don't stick to the soil as easily. Because of this, they can sometimes wash out of the soil when it rains or when fields are watered (this is called irrigation).
High levels of nitrate in drinking water can be unhealthy for babies. It can also cause too much algae to grow in lakes and ponds. This overgrowth of algae, called eutrophication, can harm fish and other water animals by using up all the oxygen. This is why the use of fertilizers, which often contain nitrogen, is carefully managed.
Denitrification: Nitrogen Returns to the Air
The nitrogen cycle isn't complete until nitrogen gas goes back into the air. This happens in places where there isn't much oxygen. Here, some bacteria can change nitrate back into nitrogen gas (N2). They do this to get energy. This process is called denitrification, and it sends nitrogen gas back into the atmosphere, starting the whole nitrogen cycle over again!
Images for kids
-
Global cycling of reactive nitrogen including industrial fertilizer production, nitrogen fixed by natural ecosystems, nitrogen fixed by oceans, nitrogen fixed by agricultural crops, NOx emitted by biomass burning, NOx emitted from soil, nitrogen fixed by lightning, NH3 emitted by terrestrial ecosystems, deposition of nitrogen to terrestrial surfaces and oceans, NH3 emitted from oceans, ocean NO2 emissions from the atmosphere, denitrification in oceans, and reactive nitrogen burial in oceans.
-
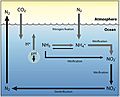
The main studied processes of the N cycle in different marine environments. Every coloured arrow represents a N transformation: N2 fixation (red), nitrification (light blue), nitrate reduction (violet), DNRA (magenta), denitrification (aquamarine), N-damo (green), and anammox (orange). Black curved arrows represent physical processes such as advection and diffusion.
See also
 In Spanish: Ciclo del nitrógeno para niños
In Spanish: Ciclo del nitrógeno para niños
 | Isaac Myers |
 | D. Hamilton Jackson |
 | A. Philip Randolph |