Eutrophication facts for kids

Eutrophication (pronounced "you-tro-fih-KAY-shun") is when a body of water, like a lake or river, gets too many nutrients. Think of it like overfeeding a plant. These extra nutrients, often from things like fertilizers or sewage, make plants and algae grow super fast.
This fast growth can cause big problems. When too many plants and algae grow, they can block sunlight for other living things underwater. When these plants die, tiny organisms called bacteria break them down. This process uses up a lot of the oxygen in the water. Without enough oxygen, fish and other aquatic animals can't breathe and might die. This can create "dead zones" where very little life can survive.
Eutrophication can happen naturally over a very long time. But often, it's caused by human activities. This is called "cultural eutrophication." It happens when things like sewage, factory wastewater, or fertilizer runoff from farms get into rivers and lakes.
Scientists and governments are working to prevent and reverse eutrophication. This includes cleaning up pollution from specific sources like pipes, and also from wider areas like farms. Some solutions even involve using shellfish or seaweed to help clean the water.
What Does Eutrophication Mean?
The word "eutrophication" comes from the Greek word eutrophos, which means "well-nourished." This name makes sense because the water becomes "over-nourished" with too many nutrients.
When water has very few nutrients, it's called oligotrophic. If it has a moderate amount, it's called mesotrophic. When it has way too many nutrients, it becomes eutrophic. This means the water quality gets worse because of too much plant and algae growth, which then decays.
People started noticing eutrophication as a big water pollution problem in lakes in Europe and North America in the mid-1900s. Important research in Canada in the 1970s showed that phosphorus was a key nutrient causing this problem in freshwater.
Why Does Eutrophication Happen?

Eutrophication happens because there are too many nutrients in the water. The most common nutrients are phosphates and nitrates.
Years ago, detergents with phosphates were a big cause. But since the 1970s, these have been mostly removed. Now, the main sources of phosphates are sewage and farming. Nitrogen pollution mostly comes from farm runoff (fertilizers and animal waste), sewage, and air pollution from burning fuels.
The amount of nutrients in a water body depends on how much comes in and how much washes out. Different places have different main nutrients. For example, phosphorus is usually the main limiting nutrient for plant growth in most freshwater. This means if you add more phosphorus, plants grow more. In marine ecosystems (oceans), nitrogen is usually the main limiting nutrient.
Human-Caused Eutrophication
Human-caused, or anthropogenic, eutrophication happens because of human activities. This problem became much bigger after chemical fertilizers were widely used in farming in the mid-1900s.
Phosphorus and nitrogen are the two main nutrients from human activity that cause this problem. They make water rich, allowing some aquatic plants, especially algae, to grow very quickly and form large "blooms." These algal blooms can block sunlight from reaching plants deeper in the water.
When these algae die, bacteria break them down. This process uses up oxygen, which can lead to anoxic (no oxygen) conditions. This lack of oxygen kills fish and other animals that need oxygen to survive. It also affects land animals that rely on the water for drinking. This can harm entire aquatic ecosystems and their food webs, leading to a loss of habitats and different species.
Sources of these extra nutrients from human activity include runoff from fertilized fields, lawns, and golf courses. Untreated sewage and wastewater also contribute. Burning fuels creates nitrogen pollution that can fall into water. Shallow waters are most easily affected. When wind and waves stir up sediments in shallow lakes, nutrients can be released from the bottom, making eutrophication worse. This poor water quality can affect drinking water, industrial uses, and fun activities like swimming.
Natural Eutrophication
Eutrophication can also be a natural process. It happens slowly over a very long time as sediments and nutrients build up naturally. This is usually from dissolved phosphate minerals and dead plant matter in the water.
Scientists who study ancient lakes (paleolimnologists) know that climate and geology also play a role in how productive lakes naturally become. The main difference between natural and human-caused eutrophication is that the natural process is extremely slow, taking thousands of years.
What Are the Effects of Eutrophication?

Effects on Nature
Eutrophication can have several effects on nature:
- More phytoplankton (tiny plants) grow.
- Changes in the types and amounts of larger water plants.
- Less dissolved oxygen in the water.
- More fish kills.
- Loss of important fish species.
Less Variety of Life
When an ecosystem gets more nutrients, primary producers like algae grow a lot. This causes an algal bloom. These blooms block sunlight from reaching plants at the bottom. They also cause big changes in the amount of oxygen in the water.
During the day, plants and algae make oxygen through photosynthesis. But at night, they use oxygen for breathing. Also, microorganisms that eat dead algae use up a lot of oxygen. When oxygen levels drop too low (called hypoxic levels), fish and other water animals can't breathe and die. This leads to "dead zones."
New Species Taking Over
Eutrophication can allow new, strong species to move in and out-compete the original species. For example, more nitrogen might help new, competitive species take over. This has happened in New England salt marshes. The common carp often thrives in eutrophic areas, which helps explain why it has spread to new places after being introduced.
Toxic Algae
Some harmful algal blooms caused by eutrophication are toxic to plants and animals. Freshwater algal blooms can be dangerous for livestock. When these algae die or are eaten, they release poisons that can kill animals and harm humans. For example, shellfish can absorb these toxins, making them unsafe for people to eat. This can cause different types of shellfish poisoning. Other marine animals can also carry these toxins.
Economic Effects
Eutrophication and harmful algal blooms can cost a lot of money. They increase water treatment costs and cause losses for commercial fishing and shellfish industries. Recreational fishing also suffers because there are fewer fish. Tourism income can drop because the water looks bad. Water treatment costs go up because the water becomes cloudy and can have bad colors or smells.
Health Impacts
Eutrophication can affect human health in two main ways: too much nitrate in drinking water and contact with toxic algae. Nitrates in drinking water can cause "blue baby syndrome" in infants. They can also react with water treatment chemicals to create harmful by-products. Direct contact with toxic algae, like from swimming or drinking affected water, can cause rashes, stomach problems, liver illness, or even breathing and nerve issues.
Eutrophication in Different Water Bodies
Freshwater Systems
When freshwater gets too many nutrients, tiny algae grow very fast, creating an algal bloom. In freshwater ecosystems, these blooms are often made of nitrogen-fixing cyanobacteria (blue-green algae). This happens when nitrogen is limited but there's still a lot of phosphorus. This extra growth can disrupt entire ecosystems and food webs, leading to a loss of habitats and different species.
When too many plants and algae die in eutrophic water, their decay uses up even more oxygen. This lack of oxygen can cause fish kills. The dead algae and other organic material sink to the bottom. There, they break down without oxygen, releasing greenhouse gases like methane and carbon dioxide.
The growth of dense algae on the surface can block sunlight from reaching deeper water. This harms plants that grow on the bottom, which affects the wider ecosystem. Eutrophication also makes rivers and lakes less enjoyable for people. It can also make drinking water treatment more difficult.
Phosphorus is often seen as the main cause of eutrophication in lakes polluted by sewage pipes. The amount of algae in lakes matches well with phosphorus levels. Studies have shown a clear link between adding phosphorus and the rate of eutrophication.
Coastal Waters
Eutrophication is common in coastal waters (near the ocean shore). Here, nitrogen is usually the main limiting nutrient, unlike in freshwater where phosphorus is key. So, nitrogen levels are more important for understanding and controlling eutrophication in saltwater. Estuaries, where fresh and saltwater mix, can be limited by both phosphorus and nitrogen. Eutrophication in estuaries often leads to low oxygen levels at the bottom, causing fish kills and harming habitats.
Human activities that add nitrogen to coastal waters include fish farming and industrial waste. Nitrogen from the air, created by burning fossil fuels, can also be a big source of nutrients in the open ocean.
Coastal waters include many marine habitats, from enclosed estuaries to the open ocean shelf. Plant growth in coastal waters depends on both nutrients and light. Light can be a problem near shore where stirred-up sediment makes the water cloudy.
Nutrients enter coastal waters from land (rivers, groundwater) and from the atmosphere. There's also a source from the open ocean, where nutrient-rich deep waters mix with surface waters. Human activity has greatly increased nitrogen and phosphorus inputs from land. The effects of eutrophication vary depending on the activities in the surrounding land and how well the water mixes with the ocean.
Effects of Coastal Eutrophication
Here are some ways coastal eutrophication shows its effects:
- More Algae: Satellite images show that the amount of chlorophyll (a measure of algae activity) is increasing in many coastal areas worldwide due to more nutrients.
- Changing Algae Types: The types of algae can change. Increases in nitrogen and phosphorus, with smaller changes in silicon, can favor certain algae over others. This leads to nuisance algal blooms in places like the North Sea and the Black Sea. Sometimes, these blooms are harmful.
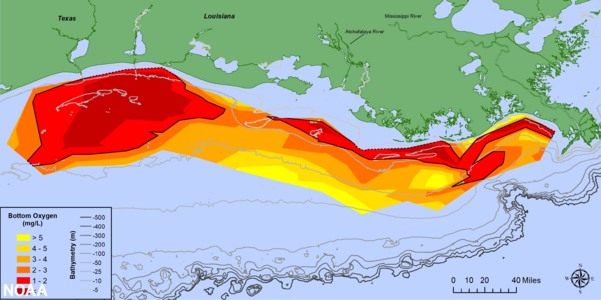
- Oxygen Loss: Oxygen depletion has happened in some coastal seas for thousands of years, like the Baltic Sea. But human activities that add organic matter can make this much worse. These areas with low oxygen have increased globally in recent decades. They are usually linked to nutrient enrichment and algal blooms. For example, in the Gulf of Mexico, a large area with seasonal low oxygen has developed since the 1950s. This is fueled by nutrients from the Mississippi River.
- Summer Kills: Low oxygen in deeper waters can lead to "summer kills." During summer, layers of water don't mix well. If too much organic matter sinks and uses up oxygen in the bottom layer, fish and other animals can die.
How Big Is the Problem?
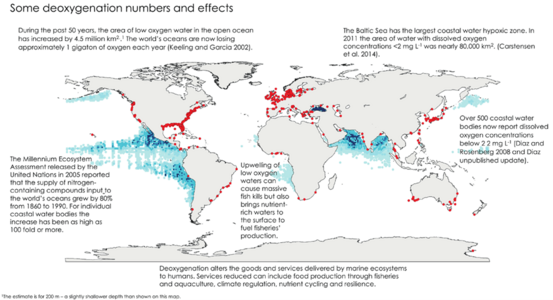
Surveys show that eutrophication is a widespread problem. For example, 54% of lakes in Asia are eutrophic, 53% in Europe, 48% in North America, 41% in South America, and 28% in Africa. In South Africa, over 60% of surveyed reservoirs were eutrophic.
The World Resources Institute has found 375 hypoxic (low oxygen) coastal zones around the world. These are common in Western Europe, the eastern and southern coasts of the US, and East Asia, especially Japan.
How Can We Prevent Eutrophication?
We can take steps to reduce eutrophication and its harmful effects.
Reducing Pollution from Sewage
One way to fix human-caused eutrophication is to treat raw sewage better. Sewage treatment plants can be improved to remove more nitrogen and phosphorus before the water is released. However, removing these nutrients is often expensive and difficult.
Laws about sewage discharge have helped a lot. It's important to provide good treatment facilities in highly populated areas, especially in developing countries, where wastewater treatment is often lacking. Using recycled wastewater safely from homes and factories is also a key step.
Reducing Nutrient Pollution from Farms
There are many ways to reduce eutrophication caused by farming. The U.S. Department of Agriculture suggests:
- Smart Nutrient Use: Farmers should apply fertilizer in the right amount, at the right time, and in the right way. Organic farming methods can sometimes reduce harmful nitrate runoff.
- Year-Round Ground Cover: Planting a cover crop keeps the ground covered even when main crops aren't growing. This stops soil and nutrients from washing away.
- Planting Field Buffers: Planting trees, shrubs, and grasses along the edges of fields can catch runoff and absorb some nutrients before they reach water bodies. These "riparian buffer zones" act like filters.
- Conservation Tillage: Reducing how often and how deeply land is tilled helps nutrients soak into the ground better.
Policies and Rules
The United Nations has goals for Sustainable Development that recognize the harm of eutrophication to marine environments. They aim to "prevent and significantly reduce marine pollution of all kinds, in particular from land-based activities, including marine debris and nutrient pollution" by 2025.
Rules and policies are important tools to reduce eutrophication. Most pollution comes from widespread sources like farms, and their effects can be lessened with good farming practices. Protecting forests can reduce erosion that carries pollutants into water. Also, using land wisely with sustainable farming methods can reduce soil runoff and nitrogen fertilizers reaching water. Better waste disposal technology also helps.
Because water bodies can affect many people across different areas, cooperation between different groups is needed. This includes state governments, water management agencies, and local communities. In the United States, a well-known effort to prevent eutrophication is the work done for the Chesapeake Bay.
How Can We Fix Eutrophication?
Reducing nutrient inputs is the most important step to restore a water body. However, it can take a long time because nutrients are stored in the bottom sediments. Lakes that have become eutrophic recover slowly, often taking many decades.
In environmental remediation (fixing environmental problems), technologies that remove nutrients include biofiltration. This uses living materials to capture and break down pollutants. Examples include green belts, riparian areas, and special treatment ponds.
Predicting Algae Blooms
The National Oceanic and Atmospheric Administration (NOAA) in the United States has created tools to predict algae blooms in places like the Great Lakes and the Gulf of Mexico. Short-term predictions help warn communities about where and how intense blooms will be. Longer-term predictions help understand bigger factors that could lead to worse blooms in the future.
Using Nature to Remove Nutrients
"Nutrient bioextraction" is a way to clean water using farmed plants and animals. This means growing and harvesting shellfish and seaweed to remove nitrogen and other nutrients from natural water bodies.
Shellfish in Estuaries
It's been suggested that oyster reefs can remove nitrogen and help those who face nitrogen emission limits. Oysters and mussels can greatly affect nitrogen levels in estuaries. Their filter-feeding helps clean water by controlling algae and storing nutrients. These nutrients can then be removed when the shellfish are harvested, or they can be buried in the sediments.
Seaweed Farming
Studies show that seaweed can help improve nitrogen levels. Seaweed aquaculture can help reduce and adapt to climate change. Seaweed, like kelp, also absorbs phosphorus and nitrogen, which helps remove too many nutrients from polluted parts of the sea. Some farmed seaweeds grow very fast and can absorb large amounts of nitrogen, phosphorus, and carbon dioxide, while producing lots of oxygen. Many believe that large-scale seaweed farming could be a good solution for eutrophication in coastal waters.
Geo-engineering
Another way to fight low oxygen/eutrophication in specific areas is to pump compressed air directly into the water. This was used to restore the Salford Docks in England. For smaller waters like fish ponds, pump aeration is a common method.
Chemical Removal of Phosphorus
Removing phosphorus can help fix eutrophication. One useful chemical is alum (aluminium sulfate). This chemical is often added to the surface of the water. It sinks to the bottom, reducing phosphorus. Such chemicals have been used worldwide to manage eutrophication and algal blooms. In a large study, alum effectively reduced phosphorus in 114 lakes for about 11 years. While it lasted longer in deep lakes (21 years) than shallow ones (5.7 years), it showed how effective alum can be.
Finland started removing phosphorus in the mid-1970s from rivers and lakes polluted by factories and cities. These efforts have been very successful, removing 90% of the phosphorus.
See also
 In Spanish: Eutrofización para niños
In Spanish: Eutrofización para niños
- Biogeochemical cycle
- Ecological Quality Ratio
- Effluent
- Nitrogen cycle
- Trophic state index
- Upland and lowland (freshwater ecology)
- Water Framework Directive
 | DeHart Hubbard |
 | Wilma Rudolph |
 | Jesse Owens |
 | Jackie Joyner-Kersee |
 | Major Taylor |