Lithium facts for kids

Lithium floating in oil
|
||||||||||||||||
| Lithium | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈlɪθiəm/ |
|||||||||||||||
| Appearance | silvery-white | |||||||||||||||
| Standard atomic weight Ar, std(Li) | [6.938, 6.997] conventional: 6.94 | |||||||||||||||
| Lithium in the periodic table | ||||||||||||||||
|
||||||||||||||||
| Atomic number (Z) | 3 | |||||||||||||||
| Group | group 1: hydrogen and alkali metals | |||||||||||||||
| Period | period 2 | |||||||||||||||
| Block | s | |||||||||||||||
| Electron configuration | [He] 2s1 | |||||||||||||||
| Electrons per shell | 2, 1 | |||||||||||||||
| Physical properties | ||||||||||||||||
| Phase at STP | solid | |||||||||||||||
| Melting point | 453.65 K (180.50 °C, 356.90 °F) | |||||||||||||||
| Boiling point | 1603 K (1330 °C, 2426 °F) | |||||||||||||||
| Density (near r.t.) | 0.534 g/cm3 | |||||||||||||||
| when liquid (at m.p.) | 0.512 g/cm3 | |||||||||||||||
| Critical point | 3220 K, 67 MPa (extrapolated) | |||||||||||||||
| Heat of fusion | 3.00 kJ/mol | |||||||||||||||
| Heat of vaporization | 136 kJ/mol | |||||||||||||||
| Molar heat capacity | 24.860 J/(mol·K) | |||||||||||||||
Vapor pressure
|
||||||||||||||||
| Atomic properties | ||||||||||||||||
| Oxidation states | +1 (a strongly basic oxide) | |||||||||||||||
| Electronegativity | Pauling scale: 0.98 | |||||||||||||||
| Ionization energies |
|
|||||||||||||||
| Atomic radius | empirical: 152 pm | |||||||||||||||
| Covalent radius | 128±7 pm | |||||||||||||||
| Van der Waals radius | 182 pm | |||||||||||||||
| Spectral lines of lithium | ||||||||||||||||
| Other properties | ||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||
| Crystal structure | body-centered cubic (bcc) | |||||||||||||||
| Speed of sound thin rod | 6000 m/s (at 20 °C) | |||||||||||||||
| Thermal expansion | 46 µm/(m⋅K) (at 25 °C) | |||||||||||||||
| Thermal conductivity | 84.8 W/(m⋅K) | |||||||||||||||
| Electrical resistivity | 92.8 nΩ⋅m (at 20 °C) | |||||||||||||||
| Magnetic ordering | paramagnetic | |||||||||||||||
| Molar magnetic susceptibility | +14.2·10−6 cm3/mol (298 K) | |||||||||||||||
| Young's modulus | 4.9 GPa | |||||||||||||||
| Shear modulus | 4.2 GPa | |||||||||||||||
| Bulk modulus | 11 GPa | |||||||||||||||
| Mohs hardness | 0.6 | |||||||||||||||
| Brinell hardness | 5 MPa | |||||||||||||||
| CAS Number | 7439-93-2 | |||||||||||||||
| History | ||||||||||||||||
| Discovery | Johan August Arfwedson (1817) | |||||||||||||||
| First isolation | William Thomas Brande (1821) | |||||||||||||||
| Main isotopes of lithium | ||||||||||||||||
|
||||||||||||||||
| 6Li content may be as low as 3.75% in natural samples. 7Li would therefore have a content of up to 96.25%. |
||||||||||||||||
Lithium is a soft, shiny silver-white metal. Its name comes from the Greek word lithos, meaning 'stone'. You can find it on the periodic table with the symbol Li.
Lithium is the third chemical element, which means it has 3 protons in its center (called the nucleus) and 3 electrons orbiting around it. Its atomic number is 3. Lithium is very reactive, meaning it easily combines with other substances. It's used in many things, like batteries and some medicines.
Contents
What is Lithium Like?
Physical Features of Lithium
Lithium is part of a group called alkali metals. When you cut it, it looks silvery and shiny. It's so soft you can easily cut it with a knife! Lithium melts at a low temperature. It's also super light, almost like wood. In fact, it's the lightest metal and the lightest element when it's a solid or liquid. Lithium is good at holding heat and lets electricity pass through it easily.
How Lithium Reacts (Chemical Properties)
Lithium loves to react with water. When it touches water, it creates hydrogen gas and a basic solution called lithium hydroxide. Because it reacts with water, lithium must be kept safe in petroleum jelly. Other similar metals like sodium and potassium can be stored in oil, but lithium is too light and would just float on top!
Lithium also reacts with halogens (like chlorine) and even nitrogen gas to form lithium nitride. If lithium touches air, it gets a black coating, which then turns into a white powder made of lithium hydroxide and lithium carbonate.
Lithium Compounds: What They Make
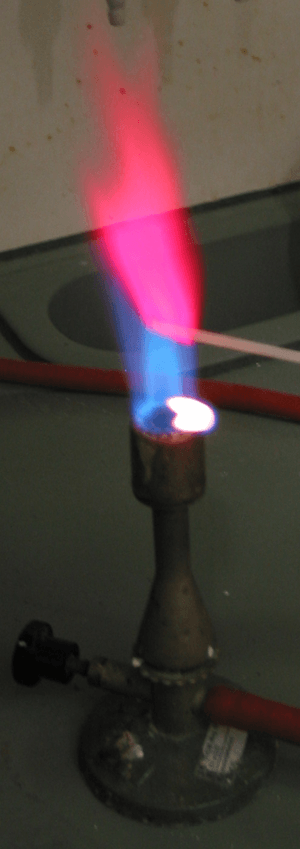
Lithium forms chemical compounds where it has a +1 oxidation state. Most of these compounds are white and don't react much. When you heat them in a flame, they glow a bright red color. They can be a little bit harmful, but most of them dissolve in water. Lithium carbonate doesn't dissolve as easily in water as some other similar compounds.
Here are some important lithium compounds:
- Lithium carbonate: Used in medicine.
- Lithium chloride: A clear, crystalline solid that makes a red flame when heated.
- Lithium hydroxide: A strong base, used to remove carbon dioxide in spacecrafts.
- Lithium nitrate: Helps other things burn (an oxidizing agent).
- Lithium nitride: A strong base.
- Lithium oxide: Dissolves in water to make lithium hydroxide.
- Lithium peroxide: Reacts with water to make oxygen.
Where is Lithium Found?
You won't find pure lithium metal in nature. It's always found as part of chemical compounds. The ocean contains a lot of lithium. Some types of granite rock also have a lot of it. Most living things, including us, have small amounts of lithium. It's also found in salty areas and in some silicate rocks.
History of Lithium
Lithium was found by Johann Arfvedson in 1817. In 1818, Christian Gmelin noticed that lithium salts made a bright red color when burned in a flame. Later, W.T. Brande and Sir Humphry Davy used electrolysis (using electricity to separate chemicals) on lithium oxide to get pure lithium metal.
At first, lithium was used in greases. Then, it became important for certain weapons. It was also used to help glass and aluminium oxide melt more easily. Today, lithium is mostly used in batteries. It was named "lithium" because it was discovered from a mineral (a "stone"), while other similar metals were first found in plants.
How Lithium is Made
Lithium is made by taking lithium chloride from natural pools and springs. This lithium chloride is then melted and put through electrolysis. This process separates it into liquid lithium metal and chlorine gas.
Uses of Lithium
Lithium as an Element
The main use for lithium metal is in batteries. It acts as an anode (a part that helps electricity flow) in lithium batteries. These batteries are more powerful than ones made with zinc. Lithium-ion batteries also use lithium, but not as a pure element.
Lithium is also used in special metal mixtures called alloys that transfer heat well. It helps make organolithium compounds, which are very strong bases used in chemistry.
Because lithium is the lightest known metal, it can be mixed with other metals like aluminium, copper, and cadmium. These mixtures create strong, lightweight metals perfect for aircraft.
Lithium in Compounds
Lithium compounds are used in some drugs called mood stabilizers. These help people with certain mental health conditions. Lithium niobate is used in radio transmitters found in cell phones. Some lithium compounds are also used to make ceramics.
Lithium chloride can absorb water from other things, making it useful as a drying agent. Some lithium compounds are also used to make soap and grease. Lithium carbonate is a medicine used to treat bipolar disorder and other mental illnesses.
Lithium in Organic Chemistry
Organolithium compounds are used to make polymers (large molecules) and fine chemicals (specialized chemicals). Many lithium compounds act as reagents (substances that cause chemical reactions) to create organic compounds. Some, like lithium aluminium hydride, are very strong bases called superbases.
Other Uses for Lithium
Lithium compounds are used to create the red color in fireworks and flares. Lithium chloride and lithium bromide are used as desiccants to dry out gas streams.
Lithium hydroxide and lithium peroxide are used to remove carbon dioxide and clean the air in spacecrafts and submarines. These compounds are also found in "oxygen candles" that provide oxygen to submarines.
Lithium aluminum hydride can be used as a solid fuel. A special type of lithium hydride containing lithium-6 was used in some powerful weapons.
Safety with Lithium
Lithium reacts with water, creating irritating smoke and heat. While it's not as dangerous as some other alkali metals, it still needs to be handled carefully. Lithium hydroxide is a very strong chemical that can cause burns.
Lithium's Isotopes
Lithium has five different isotopes. Isotopes are versions of an element that have the same number of protons but different numbers of neutrons in their nucleus. The most common isotope of Lithium in nature is 3Li7, which makes up about 92.58% of all lithium. The next most common is 3Li6, making up 7.42%. The other three isotopes are very rare. The average atomic mass of Lithium is 6.939.
Related pages
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen & alkali metals |
Alkaline earth metals | Triels | Tetrels | Pnictogens | Chalcogens | Halogens | Noble gases |
||||||||||||
| Period |
|||||||||||||||||||
| 2 | |||||||||||||||||||
| 3 | |||||||||||||||||||
| 4 | |||||||||||||||||||
| 5 | |||||||||||||||||||
| 6 | |||||||||||||||||||
| 7 | |||||||||||||||||||
Primordial From decay Synthetic Border shows natural occurrence of the element
- Ca: 40.078 — Abridged value (uncertainty omitted here)
- Po: [209] — mass number of the most stable isotope
Images for kids
-
Lithium deuteride was used as fuel in the Castle Bravo nuclear device.
See also
 In Spanish: Litio para niños
In Spanish: Litio para niños
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |