Organolithium reagent facts for kids
Organolithium compounds are special chemicals that have a direct connection, or bond, between a carbon atom and a lithium atom. They are a very important type of organometallic compound. Think of them as tiny chemical tools!
The connection between the carbon and lithium atoms is very strong. This is because carbon and lithium pull on electrons differently. This makes the carbon atom act like a strong magnet, ready to grab onto other atoms. It can also act as a powerful helper in chemical reactions.
These compounds are similar to another group of chemicals called Grignard reagents. However, organolithium compounds are much more reactive. This means they are even more eager to react with other substances.
You can buy organolithium reagents, like butyllithium, easily. Because they are so reactive, they must be handled very carefully. They need to be kept away from air, usually in a special gas like nitrogen or argon. If they touch air, they can catch fire!
Contents
What are Organolithium Compounds?
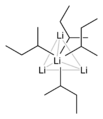
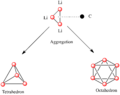
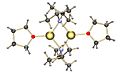
Organolithium compounds are chemicals that contain a special link. This link is directly between a carbon atom and a lithium atom. Lithium is a metal, like the one found in batteries. Carbon is a basic building block of all living things. When these two atoms connect in a certain way, they form a very useful chemical.
Why are they important?
These compounds are super important in chemistry. Scientists use them to build new and complex molecules. They are like tiny construction workers that help put different chemical pieces together. This is useful for making new medicines, plastics, and other materials.
How do they work?
The bond between carbon and lithium is very polar. This means that the electrons in the bond are not shared equally. Lithium is less "greedy" for electrons than carbon. So, the carbon atom ends up with more of the electron's negative charge.
This makes the carbon atom act like a strong "base" or "nucleophile." Imagine the carbon atom as having a strong pull. It can easily grab onto other atoms or parts of molecules. This ability makes organolithium compounds very powerful in chemical reactions. They can start reactions that other chemicals cannot.
Common Organolithium Compounds
One of the most common organolithium compounds is called butyllithium. There are different types of butyllithium, like n-butyllithium, sec-butyllithium, and tert-butyllithium. Each type has a slightly different shape. This small difference can change how they react. Scientists choose the right type for the specific reaction they want to do.
Handling Organolithium Compounds Safely
Because organolithium compounds are so reactive, they need to be handled with great care. They can react strongly with water and even the oxygen in the air.
Why are they dangerous?
- Fire Hazard: They can catch fire if exposed to air. This is why chemists use special gases like nitrogen or argon to protect them. These gases do not react with the compounds.
- Reaction with Water: They react very strongly with water. This reaction can be dangerous and produce heat.
- Corrosive: They can also be corrosive, meaning they can damage skin or other materials.
Chemists who work with these compounds wear special safety gear. This includes gloves, safety glasses, and lab coats. They also work in special enclosed areas called "gloveboxes" or "fume hoods." These tools keep the compounds away from air and protect the chemist.
Images for kids
See also
 In Spanish: Organolitio para niños
In Spanish: Organolitio para niños
 | Roy Wilkins |
 | John Lewis |
 | Linda Carol Brown |