Cubic crystal system facts for kids

In crystallography, the cubic crystal system describes a special way that crystals are shaped. Imagine a tiny building block that repeats over and over to make a crystal. This block is called a unit cell. In the cubic system, this unit cell is shaped exactly like a cube. This is one of the most common and simplest shapes found in many minerals and crystals.
Contents
What are Crystals?
Crystals are solid materials where the atoms, molecules, or ions are arranged in a very organized, repeating pattern. Think of it like building with LEGOs: each LEGO brick is placed in a specific way to create a larger structure. In crystals, these tiny building blocks are called "unit cells."
The Crystal System Idea
Scientists group crystals into seven main "crystal systems" based on the shape of their unit cells. These systems help us understand how crystals grow and why they have certain properties. The cubic system is one of these seven groups.
The Cubic Crystal System Explained
A cubic crystal system means that the basic building block (the unit cell) is a perfect cube. This means all its sides are the same length, and all its angles are 90 degrees. Because of this simple, symmetrical shape, cubic crystals often have properties that are the same in all directions.
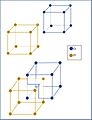
There are three main types of cubic crystals, based on where the atoms are placed within that cube-shaped unit cell:
Primitive Cubic (Simple Cubic)
The primitive cubic system is the simplest type. In this arrangement, atoms are only located at the corners of the cube-shaped unit cell. Imagine a cube with a small ball at each of its eight corners. Each corner atom is shared by eight different unit cells. This type of structure is not very common in nature, but one example is the element polonium.
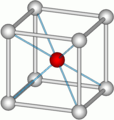
Body-Centered Cubic (BCC)
In the body-centered cubic system, atoms are at all eight corners of the cube, just like in the primitive cubic system. But there's also an extra atom right in the very center of the cube. This central atom is completely inside that one unit cell. Many metals have this structure, including iron, chromium, and tungsten.
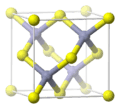
Face-Centered Cubic (FCC)
The face-centered cubic system also has atoms at all eight corners of the cube. But it adds more atoms: one atom is placed in the center of each of the cube's six faces. Each of these face-centered atoms is shared by two unit cells. This structure is very common in metals like gold, silver, copper, and aluminum. It's a very efficient way to pack atoms together.
Why Cubic Crystals are Important
The way atoms are arranged in a crystal affects its properties. For example, metals with a face-centered cubic structure (like copper or gold) are often very ductile, meaning they can be stretched into wires. This is because their atoms can slide past each other easily. Understanding these structures helps scientists and engineers create new materials with specific properties.
Examples of Cubic Minerals
Many well-known minerals crystallize in the cubic system:
- Pyrite: Often called "fool's gold," pyrite forms shiny, metallic cubes.
- Halite: This is common table salt. If you look closely at a salt crystal, you'll see it's a perfect cube.
- Garnet: These beautiful gemstones often form in shapes related to the cubic system, like dodecahedrons (12-sided shapes) or trapezohedrons (24-sided shapes), which are derived from the cubic structure.
- Fluorite: This mineral can form perfect cubic crystals in many different colors.
Images for kids
See also
 In Spanish: Sistema cristalino cúbico para niños
In Spanish: Sistema cristalino cúbico para niños
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |