Crystal structure facts for kids
In crystallography, the crystal structure is all about how the tiny pieces that make up a material (like atoms, ions, or molecules) are neatly arranged. Imagine building with LEGOs; a crystal is like a super organized LEGO structure. These arrangements happen naturally because of how chemical bonds connect the atoms. You'll find these organized patterns repeating over and over in three dimensions.
The way a crystal's structure is built, and its symmetry (meaning it looks the same when you turn it), affects many of its features. For example, it can change how the crystal breaks apart (called cleavage), how well it carries electricity, or even how light passes through it.
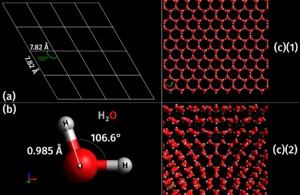
Every chemical has its own special crystal shape at the molecular level. There are many different shapes! For instance, table salt (which is Sodium chloride) always forms tiny cubes. On the other hand, copper sulfate forms a shape called triclinic. Even many metals have their own unique crystal structures. Some crystals can pack more atoms into their space than others, and these often feel heavier for their size.
Contents
What are Crystals?
Crystals are solid materials where the atoms, molecules, or ions are arranged in a very orderly, repeating pattern. Think of it like a perfectly stacked pile of oranges, but on a super tiny scale. This neat arrangement is what gives crystals their often beautiful and geometric shapes.
How Crystals Form
Crystals usually form when liquids cool down and become solid, or when substances dissolve in a liquid and then the liquid evaporates. As the material changes state, its tiny particles start to link up in the most stable way possible. This stable way often means forming a repeating pattern, which is the crystal structure. Snowflakes are a great example of crystals forming from water vapor in the air.
Why Crystal Structure Matters
The specific way atoms are arranged in a crystal is super important. It determines many of the material's properties. For example:
- Hardness: Some crystals are very hard, like diamond, because their atoms are tightly bonded in a strong structure.
- Electrical conductivity: Some materials conduct electricity well because their electrons can move easily through the crystal structure.
- Appearance: The way light interacts with a crystal's structure gives it its color, shine, and how it reflects light.
Common Crystal Shapes
There are seven main types of crystal systems, which describe the basic shapes crystals can take. Each system has different rules for how its sides and angles are arranged.
- Cubic: Like a perfect cube. Examples include table salt and diamond.
- Tetragonal: Like a cube that has been stretched or squashed along one axis.
- Hexagonal: Shaped like a hexagon, often seen in quartz and snowflakes.
- Trigonal: Similar to hexagonal but with a slightly different internal symmetry.
- Orthorhombic: Like a rectangular box, but all sides are different lengths.
- Monoclinic: Like an orthorhombic box that has been tilted on one side.
- Triclinic: The least symmetrical, with all sides and angles being different. Copper sulfate is an example.
Understanding these basic shapes helps scientists classify and study different materials.
Images for kids
-
This picture shows the crystal structure of table salt. The purple spheres are sodium atoms, and the green spheres are chloride atoms, all neatly arranged.
-
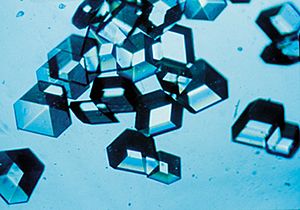
Quartz is a very common mineral, and it's a type of crystalline form of silica. You can see its beautiful crystal shape here.
See also
 In Spanish: Estructura cristalina para niños
In Spanish: Estructura cristalina para niños
 | George Robert Carruthers |
 | Patricia Bath |
 | Jan Ernst Matzeliger |
 | Alexander Miles |