Quartz facts for kids
Quick facts for kids Quartz |
|
|---|---|

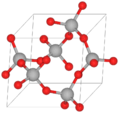
Quartz crystal cluster from Brazil
|
|
| General | |
| Category | Silicate mineral |
| Formula (repeating unit) |
SiO2 |
| Strunz classification | 4.DA.05 (oxides) |
| Dana classification | 75.01.03.01 (tectosilicates) |
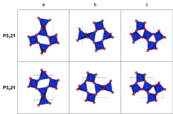
| Crystal symmetry | α-quartz: P3221 (no. 154) β-quartz: P6222 (no. 180) or P6422 (no. 181) |
| Unit cell | a = 4.9133 Å, c = 5.4053 Å; Z = 3 |
| Identification | |
| Formula mass | 60.08 g·mol−1 |
| Color | Colorless, pink, orange, white, green, yellow, blue, purple, dark brown, or black |
| Crystal habit | 6-sided prism ending in 6-sided pyramid (typical), drusy, fine-grained to microcrystalline, massive |
| Crystal system | α-quartz: trigonal β-quartz: hexagonal |
| Twinning | Common Dauphine law, Brazil law, and Japan law |
| Cleavage | {0110} Indistinct |
| Fracture | Conchoidal |
| Tenacity | Brittle |
| Mohs scale hardness | 7 – lower in impure varieties (defining mineral) |
| Luster | Vitreous – waxy to dull when massive |
| Streak | White |
| Diaphaneity | Transparent to nearly opaque |
| Specific gravity | 2.65; variable 2.59–2.63 in impure varieties |
| Optical properties | Uniaxial (+) |
| Refractive index | nω = 1.543–1.545 nε = 1.552–1.554 |
| Birefringence | +0.009 (B-G interval) |
| Pleochroism | None |
| Melting point | 1670 °C (β tridymite); 1713 °C (β cristobalite) |
| Solubility | Insoluble at STP; 1 ppmmass at 400 °C and 500 lb/in2 to 2600 ppmmass at 500 °C and 1500 lb/in2 |
| Other characteristics | Lattice: hexagonal, piezoelectric, may be triboluminescent, chiral (hence optically active if not racemic) |
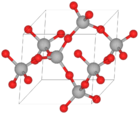
Quartz is a very common and hard mineral. It is made of silica, also known as silicon dioxide (SiO2). The tiny parts (atoms) inside quartz are linked together in a special way. Each oxygen atom is shared between two silicon atoms. This gives quartz its chemical formula of SiO2.
Quartz is the second most common mineral found in Earth's continental crust. Only feldspar is more abundant.
There are two main forms of quartz: α-quartz and β-quartz. α-quartz is the normal form found at room temperature. β-quartz forms at higher temperatures. They change from one to the other at about 573°C (1063°F). This change can cause small cracks in rocks or ceramics.
Many different types of quartz exist. Some are even used as gemstones. For a very long time, quartz varieties have been popular for making jewelry and hardstone carvings. This was especially true in Europe and Asia.
Quartz is used to define the number 7 on the Mohs scale of hardness. This scale helps us measure how hard a material is by seeing how easily it can be scratched.
Contents
- What's in a Name? The Story of "Quartz"
- Early Discoveries About Quartz
- How Quartz Crystals Grow
- Types of Quartz: By How They're Formed
- Types of Quartz: By Color
- Quartz and Electricity
- Where Quartz is Found
- How Quartz is Mined
- Other Minerals Like Quartz
- Safety When Working with Quartz
- Man-Made and Treated Quartz
- Uses of Quartz
- See also
What's in a Name? The Story of "Quartz"
The name "quartz" comes from the German word Quarz. This word was used in the 1300s. It might have come from a Polish word, twardy, or a Czech word, tvrdý, both meaning "hard."
Long ago, the Ancient Greeks called quartz krustallos. This word meant "icy cold." Some thinkers, like Theophrastus, believed quartz was a type of supercooled ice. Today, very clear quartz is sometimes called rock crystal.
Early Discoveries About Quartz
The Roman writer Pliny the Elder thought quartz was water that had frozen permanently. He noticed it was found near glaciers, not volcanoes. He also knew that quartz could split light into different colors.
In the 1600s, Nicolas Steno studied quartz. He found something amazing: no matter the size or shape of a quartz crystal, its long sides always met at a perfect 60° angle. This discovery helped start the study of crystallography.
How Quartz Crystals Grow
At room temperature, quartz crystals have a trigonal shape. Above 573°C (1063°F), they change to a hexagonal shape. The perfect quartz crystal looks like a six-sided prism with pyramid-like ends.
In nature, quartz crystals often grow together. They can be twinned (meaning two crystals grow as mirror images). They might also be distorted or mixed with other minerals. This means they don't always show their perfect shape.
Sometimes, quartz crystals form a layer inside a hollow space in a rock. This is called a druse. Geodes are great examples of this. The crystals are attached to the rock on one side. But sometimes, crystals grow freely and have two pointed ends.
The change between α-quartz and β-quartz involves a small twist of the atoms. This change causes a big shift in the crystal's size. This can lead to cracks in materials that contain quartz, especially when they are heated or cooled quickly.
Types of Quartz: By How They're Formed
Quartz varieties are often named for their color. But scientists also classify them by their tiny structure. Some quartz has crystals you can see with your eyes (macrocrystalline). Others have crystals so small you need a microscope (microcrystalline or cryptocrystalline).
| Type | Color and description | Transparency |
|---|---|---|
| Herkimer diamond | Colorless | Transparent |
| Rock crystal | Colorless | Transparent |
| Amethyst | Purple to violet | Transparent |
| Citrine | Yellow to reddish-orange or brown | Transparent |
| Ametrine | A mix of purple/violet and yellow/orange/brown | Transparent |
| Rose quartz | Pink, sometimes shows a star effect | Transparent |
| Chalcedony | Fibrous, various colors, tiny crystals. Often white or cloudy. | Translucent |
| Carnelian | Reddish orange chalcedony | Translucent |
| Aventurine | Quartz with tiny shiny bits (like mica) | Translucent to opaque |
| Agate | Multi-colored, curved or banded chalcedony | Semi-translucent to translucent |
| Onyx | Multi-colored, straight banded chalcedony | Semi-translucent to opaque |
| Jasper | Opaque, tiny crystals, usually red to brown | Opaque |
| Milky quartz | White, sometimes shows a star effect | Translucent to opaque |
| Smoky quartz | Light to dark gray, sometimes brownish or black | Translucent to opaque |
| Tiger's eye | Fibrous gold, red-brown or bluish, shows a cat's eye effect | |
| Prasiolite | Green | Transparent |
| Rutilated quartz | Contains needle-like rutile crystals inside | |
| Dumortierite quartz | Contains blue dumortierite crystals | Translucent |
| Prase | Green | Translucent |
Types of Quartz: By Color
Pure quartz is clear and colorless. It's often called rock crystal. It has been used for carvings for a long time. The different colors of quartz come from tiny amounts of other elements (impurities) inside the crystal. These impurities change how light passes through the quartz, giving it color.
Quartz types are also grouped by how big their crystals are. Macrocrystalline quartz has crystals you can see. Microcrystalline or cryptocrystalline quartz has crystals so small you need a microscope. Clear quartz is usually macrocrystalline. Cloudy or opaque quartz is often cryptocrystalline.
Chalcedony is a cryptocrystalline form of silica. It's made of tiny quartz crystals mixed with another mineral called moganite. Other opaque gemstone types of quartz include agate, carnelian, onyx, heliotrope, and jasper. These often have cool patterns or bands of color.
Amethyst
Amethyst is a beautiful purple to violet type of quartz. Big amethyst deposits are found in Brazil, Mexico, and Russia. Sometimes, amethyst and citrine grow together in one crystal. This is called ametrine. Amethyst gets its color from tiny bits of iron inside it.
Blue Quartz
Blue quartz gets its color from tiny fibers of other minerals inside it. These can be magnesio-riebeckite or crocidolite.
Dumortierite Quartz
This quartz has blue spots from dumortierite minerals inside. It can also be purple or gray. Sometimes it has light and dark color zones. It's a less common gemstone.
Citrine
Citrine is a yellow to brown type of quartz. Its color comes from tiny amounts of iron. Natural citrine is rare. Most citrine you see is actually amethyst or smoky quartz that has been heated. You can often tell the difference because heat-treated amethyst has small lines inside. Natural citrine looks more cloudy or smoky.
Brazil is a major producer of citrine. The name "citrine" comes from a Latin word meaning "yellow." It's sometimes called the "merchant's stone" because people believed it brought good luck with money. Ancient Greeks used yellow quartz for jewelry around 300 to 150 BC.
Milky Quartz
Milky quartz is the most common type of quartz that has visible crystals. It's white because of tiny bubbles of gas or liquid trapped inside when it formed. These bubbles make it less useful for clear lenses or high-quality gemstones.
Rose Quartz
Rose quartz is a lovely pink to rose-red type of quartz. Its color usually comes from tiny amounts of titanium, iron, or manganese. Some rose quartz has tiny needles inside that make a star-like effect when light shines through it. Recent studies suggest its color might also come from tiny fibers of dumortierite.
There's also a rare type of pink quartz that forms clear crystals. Its color might come from phosphate or aluminium. This type of pink quartz can fade in sunlight. The first crystals were found in Maine, USA, and Brazil.
Smoky Quartz
Smoky quartz is a gray, see-through type of quartz. It can range from almost clear to a dark brownish-gray or even black. Its color comes from natural radiation acting on tiny bits of aluminum in the crystal.
Prase
Prase is a green type of quartz. Its green color is caused by other minerals called amphiboles inside it.
Prasiolite
Prasiolite is another green type of quartz. Its green color comes from iron. It's rare to find naturally. Most prasiolite is made by heating amethyst. Natural prasiolite has been found in Brazil and Poland.
Quartz and Electricity
Quartz crystals have a special property called piezoelectric. This means they can create a small electric charge when you put pressure on them. This was discovered by Jacques and Pierre Curie in 1880.
Where Quartz is Found
Quartz is a key part of rocks like granite. It's also very common in sedimentary rocks such as sandstone and shale. You can find it in schist and gneiss, which are metamorphic rocks. Quartz is very tough and doesn't break down easily. This is why it's often found in river sediments and soils. If a rock has a lot of quartz, it means it has been through a lot of weathering.
Most quartz grows from melted rock (magma). But it can also form from hot water deep underground. Large quartz crystals can be found in pegmatites. Some can be several meters long and weigh hundreds of kilograms! The biggest single quartz crystal ever found was in Brazil. It was about 6.1 meters (20 feet) long and weighed over 88,000 pounds (40,000 kg).
How Quartz is Mined
Quartz is usually mined from open pits. Sometimes, explosives are used to reach deep pockets of quartz. More often, machines like bulldozers remove soil to find quartz veins. Then, workers use hand tools to carefully remove the crystals. It's important to avoid sudden temperature changes, which can damage the crystals.
Other Minerals Like Quartz
Quartz is part of a family of minerals made of silica (SiO2).
- Tridymite and cristobalite are forms of silica that appear at high temperatures in volcanic rocks.
- Coesite and Stishovite are denser forms of silica found in places where meteorites have hit Earth.
- Moganite is another type of silica with a different crystal structure.
- Lechatelierite is a natural glass made of silica. It forms when lightning strikes sand.
Safety When Working with Quartz
When people cut, grind, or drill natural stone that contains quartz, tiny dust particles can be released into the air. Breathing in this fine dust can be harmful. It can cause lung diseases like silicosis. This is why workers need to be careful and use protective gear.
Man-Made and Treated Quartz
Not all quartz varieties are found naturally. Some clear quartz crystals can be treated with heat or radiation to change their color. For example, most green prasiolite is made by heating amethyst. Most yellow citrine is also made by heating amethyst or smoky quartz. Even carnelian has been heat-treated for a long time to make its color deeper.
Because natural quartz crystals often have flaws, scientists make synthetic (man-made) quartz for industry. Large, perfect crystals are grown in special machines called autoclaves using a process called hydrothermal synthesis.
Quartz can also be coated with metal to give it a shiny look.
Uses of Quartz
Quartz has been important to humans for thousands of years.
- In Australian Aboriginal mythology, quartz is seen as a mystical substance.
- It has been found in ancient burial sites in Europe.
- In Prehistoric Ireland and other countries, quartz was used to make stone tools.
For a long time, different types of quartz were the most popular stones for jewelry and hardstone carvings in Europe and the Middle East. This included engraved gems, cameo gems, and fancy vases.
Scientists started trying to make quartz in labs in the mid-1800s. A German geologist, Karl Emil von Schafhäutl, made tiny quartz crystals in a pressure cooker in 1845.
Very pure natural quartz is rare and expensive. It's needed for making equipment in the semiconductor industry. One important mining spot for high-purity quartz is in North Carolina, USA.
By the 1930s, the electronics industry needed a lot of quartz crystals. During World War II, it was hard to get quartz from Brazil, which was the main source. So, countries started trying to make quartz on a large scale. After the war, many labs worked on growing big quartz crystals. By the 1950s, man-made quartz was being produced for industry. Today, almost all the quartz used in electronics is synthetic.
One of the first uses of quartz's piezoelectric property was in phonographs. Today, one of the most common uses is in crystal oscillators. These are tiny electronic devices that use quartz to keep very accurate time. The quartz clock is a common example. Quartz oscillators are also used to measure very small changes in weight.
-
A rock crystal jug from the 1500s, now in the National Museum in Warsaw.
Most of the quartz used in electronics is man-made. However, natural quartz crystals are still popular for use as gemstones. The demand for natural quartz has grown, and sometimes it is mined using very basic methods in developing countries.
See also
 In Spanish: Cuarzo para niños
In Spanish: Cuarzo para niños
- Fused quartz
- List of minerals
- Quartz fiber
- Quartz reef mining
- Quartzolite
- Shocked quartz
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |