Silicon dioxide facts for kids
Silicon dioxide, also known as silica, is a chemical compound made from silicon and oxygen. Its chemical symbol is SiO2. It's known for being very hard and has been used for a long time. You can find it in many common things like most types of glass, concrete, porcelain, and stoneware.
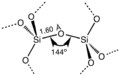
Silicon dioxide is made of one silicon atom and two oxygen atoms.
You can find silica naturally in many forms, like flint, quartz, and opal. If you breathe in silica as fine dust, it can get trapped in your lungs and cause a lung disease called silicosis or even cancer. However, if it's not in dust form, it is generally harmless.
Contents
What is Silicon Dioxide Used For?

Building and Construction
Most of the silicon dioxide used in the world (about 95%) is in the construction industry. It's a key ingredient in making concrete, which is used to build roads, bridges, and buildings.
Silica, often in the form of sand, is also used in a process called sand casting. This is where hot metal is poured into molds made of sand to create metal parts for machines and other items. Silica is good for this because it can handle very high temperatures without melting.
It is also used in a process called hydraulic fracturing (or fracking) to help get oil and gas from deep underground rocks.
Making Glass and Silicon
Silica is the main ingredient for making most types of glass. When pure silicon dioxide is melted and then cooled down quickly, it doesn't form crystals. Instead, it turns into a solid, clear material we call glass.
A very fine powder form of silicon dioxide, called fumed silica, is made by burning a chemical called silicon tetrachloride in a special flame. This creates a "smoke" of tiny SiO2 particles.
Most optical fibers, which carry internet and phone signals, are also made from very pure silica. Silica is also a main material for making many types of ceramics, like earthenware, stoneware, and porcelain.
Silicon dioxide is also used to create pure silicon. This is done by heating it in a special furnace with carbon.
In Food and Medicine
Silica is often added to food products. It helps powdered foods, like spices or coffee creamer, flow smoothly and stops them from clumping together. It can also soak up water in foods that tend to get damp. It's the main part of diatomaceous earth, which is used in filters.
You might see silica listed as E551 on food labels. It's also used to help clear up drinks like wine, beer, and juice.
In medicines, silica helps powders flow better when making tablets.
For Personal Care
In cosmetics, silica can make skin look smoother by spreading light. It also helps absorb oils.
Hydrated silica is a common ingredient in toothpaste. It acts as a gentle abrasive to help scrub away plaque from your teeth.
In Electronics
Silicon dioxide is very important in making semiconductors, which are tiny parts found in almost all electronic devices. A thin layer of silicon dioxide can be grown on a silicon surface. This layer helps protect the silicon and is used in making integrated circuits, like the chips in your phone or computer.
This process, called "surface passivation," makes sure the silicon works correctly and doesn't get damaged by air or other materials. It's a key step in making MOSFETs (metal-oxide-semiconductor field-effect transistors), which are tiny electronic switches.
Other Uses
- A special type of silica that repels water (called hydrophobic silica) is used to stop foam from forming in liquids.
- Because it can handle high temperatures, silica in fiber form is used in special fabrics for heat protection.
- Silica is used to help separate DNA and RNA in scientific experiments.
- A lightweight, porous material made from silica, called a silica-based aerogel, was used on the Stardust spacecraft to collect tiny particles from space.
- Pure silica, when cooled into a glass, can be made into fiberglass, which is used for insulation and strong materials.
Images for kids
See also
 In Spanish: Óxido de silicio(IV) para niños
In Spanish: Óxido de silicio(IV) para niños
 | Shirley Ann Jackson |
 | Garett Morgan |
 | J. Ernest Wilkins Jr. |
 | Elijah McCoy |