Solubility facts for kids
Solubility is how well a substance can dissolve in another substance. Imagine stirring sugar into water. The sugar seems to disappear, right? That's because it's dissolving! Solubility tells us how much sugar can dissolve in a certain amount of water before no more will dissolve.
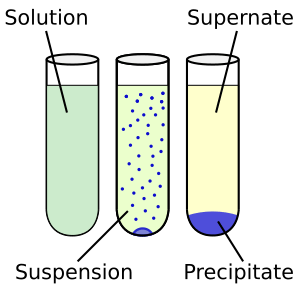
When you mix two substances and one dissolves into the other, you create a solution. If you keep adding a substance until no more can dissolve, you have a saturated solution. This means the liquid has taken in as much as it possibly can.
Sometimes, two liquids can mix together completely, no matter how much of each you add. Think of ethanol (the alcohol in hand sanitizer) mixing with water. They blend perfectly. This special ability to mix completely is called being "miscible."
Contents
What is Solubility?
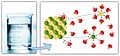
Solubility is a scientific term that describes how much of one substance can dissolve into another. It's a property of many different materials, whether they are a solid, a liquid, or a gas. When something dissolves, its tiny particles spread out evenly through the other substance.
For example, when you put salt in water, the salt crystals break apart into very tiny pieces. These pieces then spread throughout the water, making it salty. The salt is soluble in water.
Solutes and Solvents
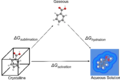
In a solution, we have two main parts:
- A solute is the substance that dissolves. It's usually the smaller amount in the mixture. For example, the sugar in sugary water is the solute.
- A solvent is the substance that does the dissolving. It's usually the larger amount. In sugary water, the water is the solvent.
Think of it like this: the solvent is the "host" that welcomes the solute "guest" to spread out and join it.
When Things Don't Dissolve
Not everything dissolves. If a substance cannot dissolve in a solvent, we say it is insoluble. For instance, if you put sand in water, the sand won't dissolve. It will just settle at the bottom. So, sand is insoluble in water.
It's important to remember that solubility doesn't depend on the size of the particles. Even large pieces of a soluble substance will eventually dissolve if given enough time and solvent.
Images for kids
See also
 In Spanish: Solubilidad para niños
In Spanish: Solubilidad para niños
 | Laphonza Butler |
 | Daisy Bates |
 | Elizabeth Piper Ensley |