Molar heat capacity facts for kids
The molar heat capacity is a scientific measurement that tells us how much energy is needed to warm up a specific amount of a substance by just one degree Celsius (or Kelvin). Think of it as how much "heat energy" a substance can hold before its temperature goes up.
This measurement is important because different materials need different amounts of energy to get hotter. For example, water needs a lot more energy to warm up than sand does. This is why sand gets hot quickly at the beach, but the ocean stays cooler!
Contents
What is Molar Heat Capacity?
Molar heat capacity helps scientists understand how substances react to heat. It's about how much energy (measured in joules) you need to add to one mole (a standard scientific amount) of a substance to make its temperature go up by one Kelvin (which is the same as one degree Celsius).
You can figure out the molar heat capacity using a simple formula: 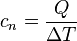 Here's what the letters mean:
Here's what the letters mean:
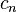 is the molar heat capacity (how much energy is needed per mole per degree).
is the molar heat capacity (how much energy is needed per mole per degree).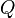 is the amount of heat energy you add (in joules).
is the amount of heat energy you add (in joules).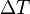 is how much the temperature changes (in Kelvin).
is how much the temperature changes (in Kelvin).
So, if you add a certain amount of heat and measure how much the temperature goes up, you can calculate the molar heat capacity!
How to Measure Molar Heat Capacity
Scientists and students often find the molar heat capacity of a substance by adding a known amount of energy to it and then measuring how much its temperature changes.
School Experiment with Water
A common experiment you might do in school to find the molar heat capacity of water involves a few simple steps:
- You take a beaker filled with water.
- You use an immersion heater (a device that heats things up and can show you exactly how much heat energy it has released).
- As the water heats up, you stir it to make sure the heat spreads evenly.
- You check the water's temperature at regular times.
By knowing the energy added by the heater and the change in water temperature, you can calculate water's molar heat capacity.
Using a Bomb Calorimeter
For more exact results, scientists use a special device called a bomb calorimeter. This device is designed to be very good at preventing heat from escaping, which helps get a super accurate measurement.
A bomb calorimeter usually has:
- A small chamber inside where a substance (like a fuel) is burned to release heat.
- This inner chamber is surrounded by water.
- The water chamber is protected by special heat-proof walls.
When the fuel burns, it releases heat into the water. Because the walls prevent heat loss, almost all the heat goes into the water, allowing for a very precise measurement of the temperature change and, therefore, the molar heat capacity.
Images for kids
-
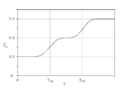
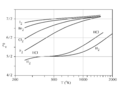
Constant-volume specific heat capacity of diatomic gases (real gases) between about 200 K and 2000 K. This temperature range is not large enough to include both quantum transitions in all gases. Instead, at 200 K, all but hydrogen are fully rotationally excited, so all have at least 52R heat capacity. (Hydrogen is already below 52, but it will require cryogenic conditions for even H2 to fall to 32R). Further, only the heavier gases fully reach 72R at the highest temperature, due to the relatively small vibrational energy spacing of these molecules. HCl and H2 begin to make the transition above 500 K, but have not achieved it by 1000 K, since their vibrational energy level spacing is too wide to fully participate in heat capacity, even at this temperature.
-
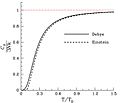
The dimensionless heat capacity divided by three, as a function of temperature as predicted by the Debye model and by Einstein's earlier model. The horizontal axis is the temperature divided by the Debye temperature. Note that, as expected, the dimensionless heat capacity is zero at absolute zero, and rises to a value of three as the temperature becomes much larger than the Debye temperature. The red line corresponds to the classical limit of the Dulong–Petit law
 | Valerie Thomas |
 | Frederick McKinley Jones |
 | George Edward Alcorn Jr. |
 | Thomas Mensah |