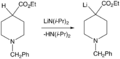Superbase facts for kids
In chemistry, a superbase is a super-strong type of base. Think of it as a chemical that is really, really good at grabbing onto tiny particles called protons.
You might know that hydroxide is the strongest base you can have in water. But superbases are much, much stronger than any base found in water. These powerful chemicals are very useful in making new substances in organic synthesis. They also help scientists understand how chemicals react in physical organic chemistry.
Contents
What are Superbases?
Superbases have been known and used by chemists since the 1850s. They are so strong that they react quickly with things like water, carbon dioxide, and even oxygen in the air. Because of this, chemists need to use special methods when working with them.
For example, they often work in an inert atmosphere. This means using a gas like nitrogen or argon that doesn't react with anything. They also keep things very cold to slow down any unwanted reactions.
How Superbases are Defined
The IUPAC (which is like the international rule-making body for chemistry) says that a superbase is simply a "compound having a very high basicity." A good example of a superbase is lithium diisopropylamide, often called LDA.
Related Chemistry Topics
Images for kids
See also
 In Spanish: Superbase para niños
In Spanish: Superbase para niños
 | May Edward Chinn |
 | Rebecca Cole |
 | Alexa Canady |
 | Dorothy Lavinia Brown |