Iodine facts for kids
Iodine is a special substance called a chemical element. Think of it as one of the basic building blocks of everything around us. Its atomic number is 53, which means each iodine atom has 53 protons. Its atomic mass is about 127. This number tells us how heavy an atom is. Iodine belongs to a group of elements called halogens on the periodic table of elements. It is also a nonmetal, meaning it doesn't conduct electricity or heat very well.
Contents
What is Iodine Like?
Physical Features
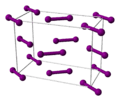
Iodine looks like a dark purple-black solid. It is a nonmetal and a halogen. When you heat iodine, it doesn't melt into a liquid. Instead, it turns directly into a bright purple gas. This process is called sublimation. The purple gas is very colorful but can be irritating if you breathe it in.
Iodine does not mix well with water. However, it can dissolve in a special water solution that already has an iodide chemical in it. It also dissolves easily in many organic liquids, like alcohol.
Chemical Features
Iodine is at the bottom of the halogen group on the periodic table. This means it is the least reactive of the halogens. Iodine is an oxidizing agent. This means it can take electrons from other atoms or molecules. But it's not as strong an oxidizer as bromine or chlorine.
When an iodine atom gains an electron from another atom, it becomes a colorless iodide ion. This process is called reduction. Iodine can react with chemicals like hydrogen sulfide to create hydriodic acid. It also reacts with bases to form other iodine compounds. Iodine can react strongly with some metals, such as aluminium. This reaction creates a lot of heat and can release harmful gases.
Iodine Compounds
Iodine can form many different chemical compounds. These compounds have iodine in various "oxidation states," which describe how many electrons it has gained or lost. The most common compounds are iodides, where iodine has gained one electron. Most organic iodine compounds are iodides.
Most iodides are colorless or reddish-yellow. They are weak reducing agents, meaning they can give electrons to other atoms. A common iodide is Potassium iodide. Iodides often turn yellow when exposed to air because they react with oxygen.
Iodine also forms compounds where it has lost electrons. For example, iodine monochloride is a red or brown liquid. Iodine trichloride is a yellow solid. Compounds like iodates (e.g., potassium iodate) are colorless solids. Iodic acid is a stable acid that contains iodine.
Compounds like periodates (e.g., sodium periodate) are also colorless solids. They are similar to perchlorates but are weaker oxidizing agents. These compounds break down into iodates when heated.
How Iodine Was Found
Iodine was discovered by a French chemist named Barnard Courtois in 1811. He was making gunpowder by burning seaweed. He used sulfuric acid to clean the ashes. One day, he accidentally poured too much sulfuric acid onto the seaweed residue. A bright purple gas appeared! This gas then turned into dark blue-black crystals on a cold surface.
Courtois didn't have enough money to fully study this new substance. So, he gave samples to other chemists. In 1813, these chemists confirmed that it was indeed a brand new element.
Where Iodine is Found
Iodine is too reactive to be found alone in nature. It always combines with other elements to form compounds. Iodine compounds are not very common in the ground. However, a mineral called caliche, found in dry deserts, contains iodate.
Iodide can also be found in seawater. Some types of Kelp (a large seaweed) have a lot of iodine in them. Iodine is one of the heaviest elements that living things need to stay healthy. If people don't get enough iodine, they can develop a condition called goitre, which causes a swollen neck.
To make sure people get enough iodine, most salt sold today has iodine added to it. This is usually in the form of iodide or iodate. People can also take iodine pills as a supplement.
How Iodine is Made
In the past, iodine was extracted from the ashes of burned kelp. But this method is not efficient enough for today's needs. Most iodine now comes from seawater.
Here's a simplified way it's made:
- Chlorine gas is added to seawater. This changes the iodide in the water into iodine.
- Air is then blown over the water to make the iodine evaporate as a gas.
- This iodine gas is then mixed with sulfur dioxide. This turns the iodine back into hydriodic acid.
- Finally, chlorine is added again to the hydriodic acid to turn it back into pure iodine. This process is repeated to make the iodine very pure.
A very pure form of iodine can also be made by mixing copper sulfate and potassium iodide. This reaction creates an unstable compound that breaks down into copper(I) iodide and pure iodine.
What Iodine is Used For
Iodine has many important uses:
- Making Chemicals: It is used as a catalyst (a substance that speeds up chemical reactions) for making acetic acid, which is found in vinegar.
- Animal Health: Animal feed often contains iodine to help animals stay healthy and get the right nutrition.
- Disinfectant: Iodine is a good disinfectant, meaning it kills germs. Tincture of iodine is a common iodine disinfectant used to clean wounds.
- Radiation Protection: Iodine can help protect people from thyroid cancer after a nuclear accident. After such an event, radioactive iodine can fill the air. This radioactive iodine can easily enter the thyroid gland in the neck and cause cancer. When people take iodine tablets, their thyroid gland fills up with non-radioactive iodine. This prevents the harmful radioactive iodine from entering.
- Medical Imaging: Iodine compounds are used in X-rays. X-rays have a hard time passing through these compounds, which helps doctors see organs and blood vessels more clearly.
- Photography: Silver iodide is used in photographic film to capture images.
- Cloud Seeding: Silver iodide is also used in cloud seeding, a process that tries to make clouds produce rain or snow.
- Food Coloring: A food coloring called Erythrosine contains iodine.
- Testing: Iodine can be used to test for certain chemicals. For example, iodine turns colorless when mixed with a reducing agent. It also turns black when mixed with starch, which is a common test for starch.
Safety with Iodine
Iodine can irritate your skin if it touches it. Its vapors can also irritate your lungs if you breathe them in. While it's less toxic than other halogens like chlorine, it's still important to be careful. Taking large amounts of iodine (like 2-3 grams) can be harmful or even deadly. Iodides are generally less toxic but can still be harmful in very large amounts.
| Periodic table | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||
Images for kids
See also
 In Spanish: Yodo para niños
In Spanish: Yodo para niños
 | Ernest Everett Just |
 | Mary Jackson |
 | Emmett Chappelle |
 | Marie Maynard Daly |