Hydrogen iodide facts for kids
Quick facts for kids Hydrogen iodide |
|
|---|---|
 |
|
 |
|
| IUPAC name | Hydrogen iodide |
|
Iodane
|
|
| Other names | Hydroiodic acid (aqueous solution) Iodine hydride |
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | C05590 |
| ChEBI | CHEBI:43451 |
| RTECS number | MW3760000 |
| SMILES | I |
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | Colorless gas |
| Odor | acrid |
| Density | 2.85 g/mL (−47 °C) |
| Melting point | |
| Boiling point | |
| approximately 245 g/100 ml | |
| Acidity (pKa) | −10 (in water, estimate); −9.5 (±1.0) 2.8 (in acetonitrile) |
| Conjugate acid | Iodonium |
| Conjugate base | Iodide |
| Refractive index (nD) | 1.466 (16 °C) |
| Structure | |
| Molecular shape | Terminus |
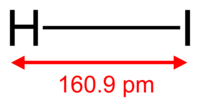
| Dipole moment | 0.38 D |
| Thermochemistry | |
| Standard molar entropy S |
206.6 J·mol−1·K−1 |
| Specific heat capacity, C | 29.2 J·mol−1·K−1 |
| Hazards | |
| Main hazards | Toxic, corrosive, harmful and irritant |
| NFPA 704 |
|
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Hydrogen fluoride Hydrogen chloride Hydrogen bromide Hydrogen astatide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Imagine a tiny molecule made of just two atoms: one hydrogen and one iodine. That's Hydrogen Iodide (HI)! It's a type of chemical compound known as a hydrogen halide. When HI gas dissolves in water, it forms a very strong liquid called hydroiodic acid. Think of it like a super-powered vinegar, but much stronger!
It's important to remember that HI itself is a gas at normal temperatures, while hydroiodic acid is the liquid solution. They can easily change from one form to the other. Scientists use HI in chemistry labs to create new substances. It's a key ingredient for getting iodine and helps other chemicals react in special ways.
Contents
What is Hydrogen Iodide?
Hydrogen iodide is a clear, invisible gas. If it touches air, especially moist air, it can react with oxygen. This reaction creates water and iodine, and you might even see a misty cloud of hydroiodic acid forming. HI loves water! A lot of HI gas can dissolve in a small amount of water, making a very strong solution. One liter of water can dissolve about 425 liters of HI gas!
What is Hydroiodic Acid?
Hydroiodic acid is simply hydrogen iodide gas dissolved in water. The strong versions you might find in a lab usually have about 48-57% HI mixed with water. This acid is one of the strongest acids known! Why is it so strong? It's because the iodide part of the molecule is quite large. This size helps the acid easily release its hydrogen part, making it very reactive.
How is Hydrogen Iodide Made?
There are several ways to create hydrogen iodide, both in factories and in chemistry labs.
Making Hydrogen Iodide in Factories
In factories, hydrogen iodide can be made by mixing iodine with a chemical called hydrazine. This reaction also creates nitrogen gas. If this process happens in water, the HI can be cleaned using a method called distillation, which separates liquids based on their boiling points.
Another way to get pure HI is by taking a solution of sodium iodide and adding a special drying agent called phosphorus pentoxide. It's important not to use strong sulfuric acid for this, because it can change the iodide into plain iodine, which isn't what we want.
Making Hydrogen Iodide in the Lab
You can also make HI by simply combining hydrogen gas and iodine gas. This method is great for making very pure HI. Scientists discovered that if you shine a specific type of light on the mixture, the reaction happens much faster! This is because the light helps the iodine molecules break apart first, making it easier for them to combine with hydrogen.
Long ago, scientists also made HI by mixing hydrogen sulfide gas with iodine in water. In a lab, chemists can also make HI by reacting iodine with phosphorus to create another compound, which then reacts with water to produce HI.
How Does Hydrogen Iodide React?
Hydrogen iodide is a very reactive chemical and takes part in many interesting reactions.
Reactions with Air and Other Chemicals
Hydroiodic acid solutions don't like air! They can easily react with oxygen in the air. This reaction changes the HI into iodine and water. The iodine then mixes with any remaining HI, forming a brownish substance. This is why older bottles of hydroiodic acid often look dark or brown.
HI can also combine with certain types of carbon compounds called alkenes. It adds itself to these molecules in a predictable way, helping to create new chemicals. Chemists use HI to change a type of alcohol into another compound called an alkyl iodide. It's like swapping out one part of a molecule for another. HI can also be used to break apart molecules called ethers. It's a useful tool for taking larger molecules and splitting them into smaller, different ones.
Historically, HI was a popular chemical for a process called 'reduction' in organic chemistry. This means it helped remove oxygen from other molecules or add hydrogen. For example, it was used to try and make certain ring-shaped molecules, and it could even change sugars into simpler compounds.
Where is Hydrogen Iodide Used?
One of the main uses of HI is in getting pure iodine from natural sources. Many places have salty water (called brine) that contains iodide. This iodide is first turned into hydroiodic acid, then changed into pure iodine, which can then be collected.
Related compounds
See also
 In Spanish: Yoduro de hidrógeno para niños
In Spanish: Yoduro de hidrógeno para niños
 | Tommie Smith |
 | Simone Manuel |
 | Shani Davis |
 | Simone Biles |
 | Alice Coachman |


