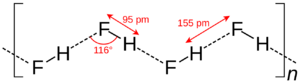Hydrogen fluoride facts for kids
Quick facts for kids Hydrogen fluoride |
|
|---|---|
 |
|
| Other names | Fluorane |
| Identifiers | |
| CAS number | |
| PubChem | |
| KEGG | C16487 |
| ChEBI | CHEBI:29228 |
| RTECS number | MW7875000 |
| SMILES | F |
|
InChI
InChI=1/FH/h1H
|
|
| Properties | |
| Molecular formula | |
| Molar mass | 0 g mol-1 |
| Appearance | colourless gas or colourless liquid (below 19.5 °C) |
| Odor | unpleasant |
| Density | 1.15 g/L, gas (25 °C) 0.99 g/mL, liquid (19.5 °C) 1.663 g/mL, solid (−125 °C) |
| Melting point | |
| Boiling point | |
| miscible (liquid) | |
| Vapor pressure | 783 mmHg (20 °C) |
| Acidity (pKa) | 3.17 (in water),
15 (in DMSO) |
| Conjugate acid | Fluoronium |
| Conjugate base | Fluoride |
| Refractive index (nD) | 1.00001 |
| Structure | |
| Molecular shape | Linear |
| Dipole moment | 1.86 D |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−13.66 kJ/g (gas) −14.99 kJ/g (liquid) |
| Standard molar entropy S |
8.687 J/g K (gas) |
| Hazards | |
| Main hazards | Highly toxic, corrosive, irritant |
| NFPA 704 |
|
| Flash point | none |
| U.S. Permissible exposure limit (PEL) |
TWA 3 ppm |
| Related compounds | |
| Other anions | Hydrogen chloride Hydrogen bromide Hydrogen iodide Hydrogen astatide |
| Other cations | Sodium fluoride Potassium fluoride Rubidium fluoride Caesium fluoride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Hydrogen fluoride (also known as fluorane) is a chemical compound made of hydrogen and fluorine. Its chemical formula is HF. It is usually a colorless gas or a clear liquid when it is colder than 19.5 degrees Celsius. It has a strong, unpleasant smell.
Hydrogen fluoride is very important in many industries. It is the main way we get fluorine, which is used to make many useful things. For example, it helps create pharmaceuticals (medicines) and polymers like polytetrafluoroethylene (PTFE), which you might know as Teflon. It is also used in the petrochemical industry to make special acids.
Because of strong connections between its molecules, called hydrogen bonds, hydrogen fluoride boils at a much higher temperature than other similar compounds. This is quite unusual for such a small molecule.
It is extremely important to know that hydrogen fluoride is very dangerous. It can cause severe burns and other serious health problems if it touches your skin or if you breathe it in. When it mixes with moisture, it forms hydrofluoric acid, which is also very corrosive.
Contents
Discovering Hydrogen Fluoride
People knew about hydrofluoric acid in the glass industry even before the late 1700s. In 1771, Carl Wilhelm Scheele, a Swedish chemist, made large amounts of this acid.
Later, a French chemist named Edmond Frémy (who lived from 1814 to 1894) is recognized for discovering hydrogen fluoride (HF). He found it while he was trying to isolate pure fluorine.
How Hydrogen Fluoride Works
Hydrogen fluoride molecules are made of one hydrogen atom and one fluorine atom. In its gas form, these molecules often stay as pairs.
When hydrogen fluoride is a liquid, its molecules link together in chains. They do this using strong connections called hydrogen bonds. These bonds are why liquid HF has a relatively high boiling point, around 19.5 °C. This is much higher than other similar compounds like hydrogen chloride, which boil at much colder temperatures.
When HF dissolves in water, it forms hydrofluoric acid. This acid is very corrosive and can cause serious harm.
Making Hydrogen Fluoride
Most hydrogen fluoride is made by reacting sulfuric acid with a mineral called fluorite. Fluorite is mostly calcium fluoride. This reaction creates hydrogen fluoride gas and calcium sulfate.
- Chemical Reaction: Calcium fluoride + Sulfuric acid → Hydrogen fluoride + Calcium sulfate
About 20% of the hydrogen fluoride made also comes as a byproduct from making fertilizers. In this process, a substance called hexafluorosilicic acid is produced, which can then be turned into hydrogen fluoride.
Uses of Hydrogen Fluoride
Hydrogen fluoride is used in many important ways, often in its pure form rather than as an acid.
Making Fluorine Compounds
HF is a key ingredient for making many organofluorine compounds. These are special chemicals that contain fluorine. For example, it is used to make tetrafluoroethylene (TFE), which is the main ingredient for Teflon. Teflon is famous for its non-stick properties in cookware.
HF also helps in a process called electrochemical fluorination. Here, HF is used to replace hydrogen atoms in organic compounds with fluorine atoms, creating new materials.
Producing Metal Fluorides and Fluorine Gas
Hydrogen fluoride is essential for making aluminium. It is used to create a substance called cryolite, which is needed to extract aluminum metal from its ore using electricity.
It is also the starting material for making pure fluorine gas (F2). This is done by passing electricity through a mixture of HF and potassium bifluoride. Pure fluorine gas is used in many specialized industrial processes.
As a Catalyst
In oil refineries, hydrogen fluoride acts as a catalyst. A catalyst is a substance that speeds up a chemical reaction without being used up itself. HF helps in making high-octane petrol (gasoline) components, which makes fuel burn more efficiently.
As a Solvent
Hydrogen fluoride is an excellent solvent, meaning it can dissolve many substances. It can even dissolve complex molecules like proteins and carbohydrates. This is because of its strong hydrogen bonding abilities.
Safety and Health Information
Hydrogen fluoride is extremely dangerous and must be handled with the greatest care. It is a powerful contact poison and highly corrosive.
- Skin Contact: If it touches your skin, it can cause very severe burns that might not hurt right away but can become very serious later.
- Eye Contact: It can seriously harm your eyes, potentially causing permanent damage.
- Breathing It In: Breathing in hydrogen fluoride gas can cause severe internal problems, especially in the lungs. It can be very dangerous and even deadly if not treated quickly.
If anyone comes into contact with hydrogen fluoride, they need immediate medical attention. Always follow strict safety rules when working with this chemical.
See also
 In Spanish: Fluoruro de hidrógeno para niños
In Spanish: Fluoruro de hidrógeno para niños