Hydrogen bond facts for kids
A hydrogen bond is a special kind of connection between molecules. It's like a weak magnet that pulls certain atoms together. This bond always involves a hydrogen atom. That's why it's called a hydrogen bond!
It happens when a hydrogen atom, which is already linked to a "greedy" atom (like oxygen or nitrogen), gets attracted to another "greedy" atom in a nearby molecule. These "greedy" atoms are called electronegative because they pull electrons towards themselves.
Contents
What is a Hydrogen Bond?
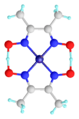
A hydrogen bond is a type of dipole-dipole bond. Think of molecules as having tiny positive and negative ends, like a small magnet. When a hydrogen atom is connected to a very electronegative atom (like oxygen, nitrogen, or fluorine), the hydrogen atom gets a slightly positive charge. The electronegative atom gets a slightly negative charge.
This slightly positive hydrogen atom is then attracted to a slightly negative atom on a different molecule. This attraction is the hydrogen bond. It's not as strong as the bonds inside a molecule, but it's strong enough to make a big difference.
Where Do Hydrogen Bonds Occur?
Hydrogen bonds can happen in two main ways:
- Between molecules (called intermolecularly): This is when hydrogen bonds form between different molecules. For example, water molecules stick together this way.
- Within a single molecule (called intramolecularly): This is when different parts of the same large molecule are held together by hydrogen bonds. This is important for the shape of complex molecules like proteins or DNA.
How Strong Are Hydrogen Bonds?
Hydrogen bonds are stronger than very weak forces called van der Waals forces. But they are weaker than the strong bonds that hold atoms together inside a molecule. These stronger bonds include covalent bonds, ionic bonds, and metallic bonds.
Even though they are weaker, hydrogen bonds are very important. They play a huge role in how many substances behave.
Why Are Hydrogen Bonds Important?
Hydrogen bonds are super important for many things around us, especially for life!
Water's Special Properties
Hydrogen bonds are what make water so unique. They are responsible for water's high boiling point (100 °C). Without hydrogen bonds, water would boil at a much lower temperature, and life as we know it might not exist!
Because hydrogen bonds make water molecules stick together so well, they also help other molecules dissolve in water. If a molecule can form hydrogen bonds with water, it will mix easily. This is why sugar dissolves in water.
Life and Materials
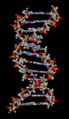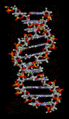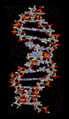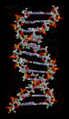
Hydrogen bonds are crucial for the structure of DNA, which carries our genetic information. They hold the two strands of the double helix together, like rungs on a ladder.
They also help give proteins their specific shapes, which is essential for them to do their jobs in our bodies. In materials science, hydrogen bonds are used to create strong materials like Kevlar, which is used in bulletproof vests.
Images for kids
-
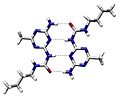
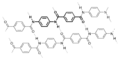
An example of intermolecular hydrogen bonding in a self-assembled dimer complex. The hydrogen bonds are represented by dotted lines.
-
The structure of part of a DNA double helix
See also
 In Spanish: Enlace de hidrógeno para niños
In Spanish: Enlace de hidrógeno para niños
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |