Covalent bond facts for kids
A covalent bond is a special connection between two atoms. It happens when atoms share their tiny outer particles called electrons. This type of bond usually forms between two non-metal atoms. A great example is water (H2O). Here, a hydrogen atom (H) and an oxygen atom (O) share electrons to form a water molecule.
Atoms have electrons orbiting around their center, like planets around a star. These electrons are arranged in layers called electron shells. The electrons in the outermost shell are called valence electrons. These are the ones involved in making bonds. For most atoms, a full outer shell has eight electrons. But for very small atoms like hydrogen, a full outer shell only needs two electrons.
The number of electrons in an atom depends on the number of protons in its center. Electrons orbit the atomic center in specific paths. The first electron shell can hold up to two electrons. The shells after that can usually hold up to eight electrons. Covalent bonds form when atoms share their valence electrons to make their outer shells full and stable.
Imagine an atom with seven electrons in its outer shell. It wants one more electron to be stable. If it gets close to another atom that has one electron it can share, they might form a bond. The shared electron will then orbit both atoms. This sharing makes both atoms more stable and holds them together. This connection is called a covalent bond. Breaking this bond needs the same amount of energy that was released when it formed.
A water molecule (H2O) is a perfect example of covalent bonding. It has one oxygen atom and two hydrogen atoms. The oxygen atom shares one electron with each hydrogen atom. This sharing makes the water molecule a "polar" molecule. This means its electrical charge is not spread out evenly. One side of the molecule has a slight positive charge, and the other has a slight negative charge. This slight difference in charge helps water do many amazing things.
Different Kinds of Covalent Bonds
Covalent bonds can be different strengths and types depending on how the electrons are shared.
- Sigma (σ) bonds are the strongest covalent bonds. They happen when electron clouds overlap directly, head-on. A single bond between two atoms is usually a sigma bond.
- Pi (π) bonds are weaker. They form when electron clouds overlap sideways.
- A double bond between two atoms has one sigma bond and one pi bond.
- A triple bond has one sigma bond and two pi bonds.
Covalent bonds are generally weaker than ionic bonds. They also have lower melting points. Materials with covalent bonds are usually not good at conducting electricity or heat.
How Long Are Covalent Bonds?
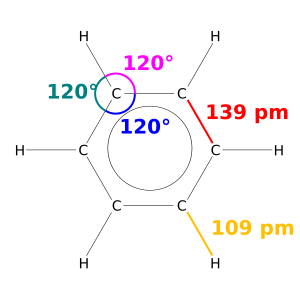
In chemistry, bond length is how we measure the size of a covalent bond. Since molecules are incredibly tiny, bond lengths are measured in picometers. A picometer is one millionth of a billionth of a meter!
The way molecules behave is mostly explained by their bonds. And these bonds are created by how their electrons are arranged.
Related pages
See also
 In Spanish: Enlace covalente para niños
In Spanish: Enlace covalente para niños
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |

