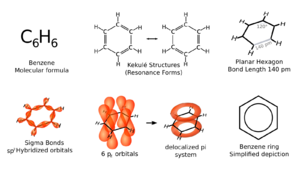Benzene facts for kids

Benzene, also known as benzol, is a special chemical compound. Its formula is C6H6. It is a clear liquid that can catch fire easily. It also has a sweet smell.
Benzene molecules are shaped like a ring. This ring has six carbon atoms. Each carbon atom is connected to one hydrogen atom. It is an aromatic compound. This means the atoms in its ring are bonded in a special way.
Benzene can cause cancer, so it must be handled with care. It is used in many everyday things. You can find it in gasoline and plastics. It's also used to make synthetic rubber and dyes. Benzene is also a strong solvent, which means it can dissolve many other chemicals.
Benzene is naturally found in crude oil. Because of this, it is also present in gasoline. Many medicines also contain parts made from benzene.
Structure of Benzene
Benzene has special features called aromaticity. All six carbon atoms in its ring lie on a flat surface. To explain all the bonds in this six-carbon ring, chemists thought it had alternating double carbon bonds.
If benzene had three double bonds, some sides of its ring would be shorter than others. But, X-ray diffraction shows that all six carbon-carbon bonds in benzene are the same length. They are about 140 picometres (pm) long. This length is longer than a typical double bond (135 pm). But it is shorter than a single bond (147 pm).
This means the bonds are somewhere in between. Scientists often show benzene with a circle inside a hexagon. This circle shows that the electrons are shared evenly around the whole ring. This sharing makes benzene very stable. This stability helps explain its unique properties.
In organic chemistry, the carbon atoms in diagrams are often not labeled. Each carbon atom in benzene shares an electron. These electrons form special "clouds" above and below the benzene ring.
In 1986, some chemists disagreed with this idea. They published their thoughts in the journal Nature. They suggested that the electrons in benzene might be fixed to certain carbon atoms. They thought benzene's aromatic properties came from something called spin coupling. Other scientists supported this idea in 1987. But, many chemists still use the idea of shared electrons.
Benzene parts are very common in organic molecules. There are even special Unicode symbols for them. One symbol is U+232C (⌬) for the three double bonds. Another is U+23E3 (⏣) for the shared electron version.
Images for kids
See also
 In Spanish: Benceno para niños
In Spanish: Benceno para niños
 | Isaac Myers |
 | D. Hamilton Jackson |
 | A. Philip Randolph |