Ionic bond facts for kids
An ionic bond is a strong connection between atoms that have become charged. It happens when one atom gives away one or more of its electrons to another atom. This makes both atoms turn into ions, which are charged particles. Then, these oppositely charged ions pull strongly on each other, forming a bond.
Imagine a metal atom and a non-metal atom. The metal atom likes to give away electrons. When it loses an electron, it becomes a positive cation. The non-metal atom likes to gain electrons. When it takes an electron, it becomes a negative anion. Because one is positive and the other is negative, they are strongly attracted to each other. This attraction is what we call an ionic bond.
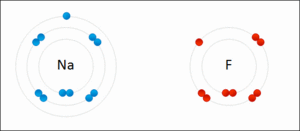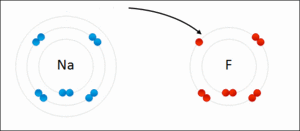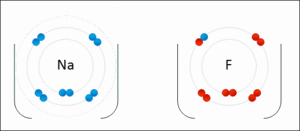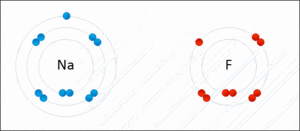
A common example is how table salt, sodium chloride (NaCl), is formed. Sodium (Na) is a metal, and chlorine (Cl) is a non-metal.
- A sodium atom gives away one electron. It becomes a positive sodium ion (Na+).
- A chlorine atom takes that electron. It becomes a negative chloride ion (Cl-).
These positive sodium ions and negative chloride ions then stick together very strongly.
Contents
What Makes Ionic Compounds Special?
Ionic compounds are substances made with ionic bonds. They have some unique features:
Crystal Structure
Ionic compounds form a special 3D shape called a giant ionic crystal lattice. Imagine a huge, repeating pattern of positive and negative ions all connected together. This structure is very strong and organized.
Dissolving in Water
Many ionic compounds can dissolve in water. When they are in water, the water molecules pull the ions apart from their lattice structure. The ions then spread out in the water. This is why salt dissolves in water.
Conducting Electricity
Ionic compounds do not conduct electricity when they are solid. This is because their ions are stuck in the crystal lattice and cannot move around. However, if you melt an ionic compound or dissolve it in water, the ions become free to move. When they can move, they can carry an electric charge, so the substance will conduct electricity.
High Melting and Boiling Points
Ionic compounds have very high melting and boiling points. This is because the electrostatic forces of attraction between the positive and negative ions are extremely strong. It takes a lot of heat energy to break these strong bonds and make the substance melt or boil.
See also
 In Spanish: Enlace iónico para niños
In Spanish: Enlace iónico para niños
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |