Vapor pressure facts for kids
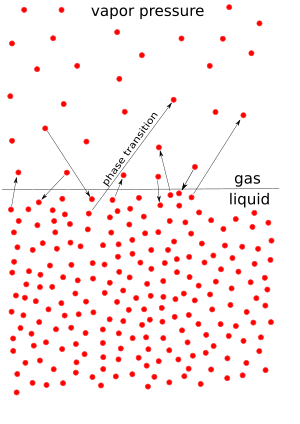
Vapor pressure is the pressure that a vapor creates when it is perfectly balanced with its liquid (or solid) form in a sealed container. This balance happens at a certain temperature. Think of it like a tiny battle: some liquid molecules are always trying to escape into the air as vapor, while some vapor molecules are trying to return to the liquid. When the number of molecules escaping equals the number returning, we have vapor pressure.
Vapor pressure tells us how quickly a liquid will turn into a gas. If a liquid has a high vapor pressure, it means many of its molecules are easily escaping into the air. This liquid will evaporate very fast! Substances that evaporate quickly at normal temperatures are called volatile (say: VOH-luh-til).
As a liquid gets warmer, its tiny molecules move around much faster. This extra movement is called kinetic energy. When molecules move faster, more of them have enough energy to break away from the liquid and become vapor. This means that as the temperature goes up, the vapor pressure also goes up.
Contents
What is Vapor Pressure?
Vapor pressure is the force that vapor molecules push with. Imagine a bottle of water with a lid on it. Some water molecules will turn into a gas (vapor) and float above the liquid. These vapor molecules bounce around inside the bottle, hitting the walls and the surface of the water. This bouncing creates pressure. That's vapor pressure!
How Does Temperature Change Vapor Pressure?
Temperature is a big deal for vapor pressure. When you heat a liquid, you give its molecules more energy. They start to zoom around much faster. This makes it easier for them to escape from the liquid and become vapor.
Think of it like a crowded room. If everyone is moving slowly, few people will leave. But if everyone is running around, more people will bump into the door and escape! The more molecules that escape into vapor, the higher the vapor pressure becomes. This is why water boils at a lower temperature high up on a mountain. The air pressure is lower, so the water needs less vapor pressure to boil.
Why is Vapor Pressure Important?
Vapor pressure helps us understand many things. It explains why some liquids, like nail polish remover, dry very quickly. They have a high vapor pressure. It also helps scientists and engineers design things like engines and chemical processes.
For example, in a cloud chamber, vapor pressure is used to show tiny particles. If the vapor pressure is too high, the vapor turns back into liquid. This forms tiny droplets around the path of the particles, making them visible!
Images for kids
-
If vapor pressure gets too high, the vapor turns into liquid. This is how cloud chambers work, showing paths of tiny particles.
See also
 In Spanish: Presión de vapor para niños
In Spanish: Presión de vapor para niños
 | Kyle Baker |
 | Joseph Yoakum |
 | Laura Wheeler Waring |
 | Henry Ossawa Tanner |