Octane facts for kids
Octane is an organic compound that is very important for us. It's a chemical made mostly of carbon and hydrogen atoms. You might not know it, but octane is a big part of the gasoline that powers cars and other machines. It's what helps release a lot of energy when gasoline burns.
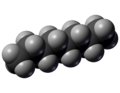
Octane is a clear liquid that smells a bit like gasoline. It's a type of chemical called an alkane, which means it has a simple chain of eight carbon atoms linked together, with hydrogen atoms attached to them.
Contents
What is Octane Used For?
Octane is mainly used as a key part of gasoline. When you hear about "high octane" fuel, it means the fuel is better at resisting something called "engine knocking." Engine knocking happens when fuel burns unevenly in an engine, which can damage it.
Why is High Octane Fuel Better?
High octane fuel burns more smoothly and efficiently. This helps car engines run better and last longer. It's especially important for high-performance cars.
Properties of Octane
Octane is a colorless liquid. It has a density of about 0.703 grams per cubic centimeter. This means it's lighter than water.
How Does Octane React?
Octane is very flammable. It can easily catch fire and burn. This is why it's used as a fuel. It boils at about 125–126 °C (257–259 °F) and freezes at about -57 °C (-71 °F).
Safety Information
Octane is a dangerous chemical if not handled carefully. It is highly flammable, meaning it can easily catch fire. It can also irritate your skin and eyes. Breathing in a lot of octane vapor can make you feel dizzy or sleepy.
Handling Octane Safely
Because it's so flammable, octane should always be kept away from heat, sparks, and open flames. It should be stored in a well-ventilated area. If you ever come across it, remember to be careful and follow safety rules.
Related Chemicals
Octane belongs to a family of chemicals called alkanes. Other alkanes include:
- Heptane
- Nonane
These chemicals are similar to octane but have different numbers of carbon atoms in their chains.
Images for kids
See also
 In Spanish: Octano para niños
In Spanish: Octano para niños
 | James Van Der Zee |
 | Alma Thomas |
 | Ellis Wilson |
 | Margaret Taylor-Burroughs |