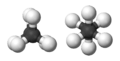Alkane facts for kids
Alkanes are special chemical compounds. They are made only from carbon and hydrogen atoms. Think of them as the simplest building blocks in the world of organic chemistry!
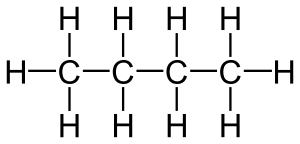
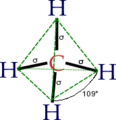
The smallest alkane is called methane. It has just one carbon atom connected to four hydrogen atoms. Larger alkanes have two or more carbon atoms linked together in a chain. What makes alkanes unique is that their carbon atoms are joined by single bonds. This is different from other similar compounds like alkenes, which have double bonds.
You can figure out the formula for any alkane using a simple rule: CnH2n+2. Here, 'n' stands for the number of carbon atoms.
Here are some common alkanes you might hear about:
|
|
Where Do Alkanes Come From?
Most alkanes we use come from crude oil. Crude oil is a thick, dark liquid found deep underground. It's a natural resource that we get by drilling into the Earth.
Crude oil isn't just one type of alkane. It's actually a mix of many different alkanes. These alkanes have different numbers of carbon atoms in their chains.
To get pure alkanes from crude oil, scientists use a method called Fractional distillation. This process separates the different alkanes based on their boiling points. It helps us get purer samples of specific alkanes for various uses.
Images for kids
-
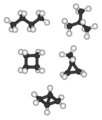
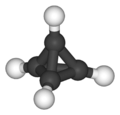
C4 alkanes and cycloalkanes (left to right): n-butane and isobutane are the two C4H10 isomers; cyclobutane and methylcyclopropane are the two C4H8 alkane isomers. Bicyclo[1.1.0]butane is the only C4H6 alkane and has no alkane isomer; tetrahedrane (below) is the only C4H4 alkane and so has no alkane isomer.
-
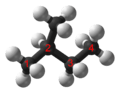
Monobromination of propane
-
Extraction of oil, which contains many distinct hydrocarbons including alkanes
-
Methanogenic archaea in the gut of this cow are responsible for some of the methane in Earth's atmosphere.
-
An oil refinery at Martinez, California.
See also
 In Spanish: Alcano para niños
In Spanish: Alcano para niños
 | Shirley Ann Jackson |
 | Garett Morgan |
 | J. Ernest Wilkins Jr. |
 | Elijah McCoy |