Fractional distillation facts for kids
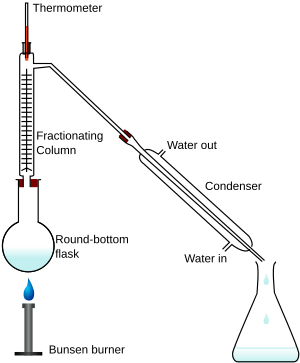
Fractional distillation is a way to separate different liquids from a mixture. Imagine you have a mix of liquids, like water and alcohol. They all have different boiling points. This means they turn into a gas at different temperatures.
Fractional distillation uses these different boiling points. You heat the mixture. Each liquid turns into a gas (evaporates) at its own temperature. Then, these gases cool down and turn back into liquid (condense). Each liquid is collected separately. This way, you get pure liquids from the original mixture.
What is Fractional Distillation?
This process is a special kind of distillation. It is used when the liquids in a mixture have boiling points that are quite close. For example, if their boiling points are less than 25°C apart. If the boiling points are very different (more than 25°C apart), a simpler method called simple distillation is used instead.
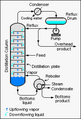
The process happens in a tall tube called a fractionating column. When you heat the mixture, the liquids start to boil. They turn into vapor at different times. As the vapors rise in the column, they cool and condense. Each pure liquid is then collected in its own container.
How is it Used in Industry?
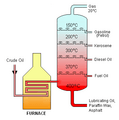
Fractional distillation is very important in many industries. A great example is how petroleum (crude oil) is processed. Crude oil is a mix of many different chemicals. These chemicals are called fractions.
In a refinery, crude oil is heated up. It then goes into a very tall fractionating column. This column is cooler at the top and hotter at the bottom. Different parts of the crude oil have different boiling points.
- Heavy parts, which have high boiling points, turn into liquid lower down the column.
- Lighter parts, which have lower boiling points, rise higher up the column before they cool and turn into liquid.
This process separates crude oil into useful products. These include gasoline, diesel, and other fuels.
Images for kids
-
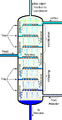
Crude oil is separated into fractions by fractional distillation. The fractions at the top of the fractionating column have lower boiling points than the fractions at the bottom. All of the fractions are processed further in other refining units.
See also
 In Spanish: Destilación fraccionada para niños
In Spanish: Destilación fraccionada para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |