Fractionating column facts for kids
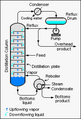
A fractionating column is a special device used in chemistry. It helps to separate different liquids that have different boiling points. Imagine you have a mix of two liquids, like water and alcohol. A fractionating column can separate them by heating the mixture. The liquid that boils at a lower temperature turns into a gas first. This gas then travels up the column and cools down, turning back into a liquid. This process is called fractional distillation. Large fractionating columns used in factories are often called fractionating towers.
Contents
What is a Fractionating Column Used For?
Fractionating columns are super important tools. They are used to separate mixtures of liquids. These liquids must have different boiling points. For example, if you have a mix of water and ethanol, water boils at 100°C and ethanol boils at 78°C. A fractionating column can separate them.
Separating Crude Oil
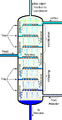
One of the biggest uses for these columns is in oil refineries. Crude oil is a mix of many different liquids. These liquids include gasoline, diesel, jet fuel, and more. Each of these has a different boiling point. The fractionating column heats the crude oil. Then, it separates these valuable parts. This process helps us get the fuels we use every day.
In the Lab
Chemists also use smaller fractionating columns in their labs. These are often made of glass. They help scientists purify chemicals. This is important for making new medicines or materials.
How Does a Fractionating Column Work?
A fractionating column works by using heat and cooling. It takes advantage of the different boiling points of liquids.
Heating the Mixture
First, the mixture of liquids is heated. This happens at the bottom of the column. The liquid with the lowest boiling point turns into a gas first. This gas is called vapor.
Traveling Up the Column
The vapor then rises up the column. As it goes up, it cools down. The column has special parts inside. These parts help the vapor cool and condense. Condense means turning back into a liquid.
Trays and Plates
Inside the column, there are many trays or plates. These trays help the vapor and liquid mix. As the vapor rises, it gets purer. The liquid that condenses on the trays flows back down. This process happens over and over. Each time, the vapor gets richer in the lower-boiling liquid.
Collecting the Separated Liquids
Different liquids condense at different heights in the column. The liquids with lower boiling points go higher up. The liquids with higher boiling points stay lower. This allows different liquids to be collected separately. They come out at different points along the column.
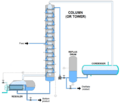
Types of Fractionating Columns
There are two main types of fractionating columns. They are used for different purposes.
Laboratory Columns
These columns are smaller. They are usually made of glass. They are used in science labs. An example is the Vigreux column. It has many small indentations inside. These help the vapor and liquid mix well.
Industrial Columns
These columns are very large. They are often made of steel. You can see them in oil refineries or chemical plants. They can be many stories tall. These giant columns can process huge amounts of liquid every day. They are essential for making many products we use.
See also
 In Spanish: Columna de fraccionamiento para niños
In Spanish: Columna de fraccionamiento para niños
Images for kids
 | Shirley Ann Jackson |
 | Garett Morgan |
 | J. Ernest Wilkins Jr. |
 | Elijah McCoy |