Ethane facts for kids
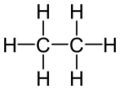
Ethane is a simple organic compound with the chemical formula C2H6. This means it has two carbon atoms and six hydrogen atoms. At normal temperatures and pressures, ethane is a gas that you can't see or smell.
It's found in natural gas and is also made as a byproduct when petroleum (crude oil) is refined. Ethane is very important in the chemical industry, mainly used to create ethylene through a process called steam cracking.
Be careful with ethane! At room temperature, the gas can easily catch fire, and it can even explode if it mixes with air. If ethane is in its liquid form, touching it can cause very serious frostbite.
Contents
What is Ethane?
Ethane is part of a family of chemicals called alkanes. These are simple hydrocarbons, meaning they are made only of hydrogen and carbon atoms. Ethane is the second simplest alkane, right after methane (which has one carbon atom).
Because it's a gas at normal temperatures, ethane is often stored and transported as a very cold liquid under pressure.
Where Does Ethane Come From?
Most of the ethane we use comes from natural gas fields. When natural gas is taken out of the ground, it's a mix of different gases, including methane (the main part of natural gas), propane, butane, and ethane. Ethane is separated from this mixture.
It's also produced when oil refineries process petroleum. This process breaks down larger molecules in crude oil into smaller, more useful ones, and ethane is one of the products.
How is Ethane Used?
The biggest use for ethane is to make another important chemical called ethylene. This is done through a process called "steam cracking." In steam cracking, ethane gas is heated to very high temperatures (around 800-900 °C or 1,472-1,652 °F) in the presence of steam. This heat breaks the chemical bonds in ethane, turning it into ethylene.
Ethylene is a building block for many plastics and other chemicals. For example, it's used to make polyethylene, which is a common plastic found in things like plastic bags, bottles, and toys. So, ethane helps create many everyday items!
Safety Information
Ethane is a flammable gas. This means it can easily catch fire and burn. If ethane gas mixes with air, it can form an explosive mixture. This is why it's handled with great care in industrial settings.
When ethane is cooled down enough, it turns into a liquid. This liquid is extremely cold (its boiling point is about -88.5 °C or -127.3 °F). If liquid ethane touches your skin, it can cause severe frostbite, similar to a burn, because it freezes the tissue very quickly.
Images for kids
-
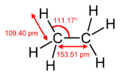
Molecular geometry of ethane based on rotational spectroscopy.
-
A photograph of Titan's northern latitudes. The dark features are hydrocarbon lakes containing ethane.
See also
 In Spanish: Etano para niños
In Spanish: Etano para niños
 | Georgia Louise Harris Brown |
 | Julian Abele |
 | Norma Merrick Sklarek |
 | William Sidney Pittman |