Molecular geometry facts for kids

Molecular geometry is all about the shape of molecules. Think of it like how building blocks are put together. It describes how atoms are arranged in a three-dimensional space. This special arrangement is super important because it affects many things about a molecule, like how it acts or reacts with other molecules.
Understanding Molecular Shapes
Molecules come in many different shapes. These shapes depend on two main things:
- The number of atoms in the molecule.
- The number of electron pairs around the central atom.
These factors also decide the angles between the bonds. For example, a molecule with a straight, "linear" shape will have a bond angle of 180 degrees. This means the atoms are spread out as far as possible in a straight line.
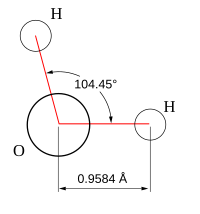
Bent Molecules
Some molecules, like water, have a "bent" shape. Their bond angle is less than 180 degrees. This happens because the central atom has extra electron pairs that are not part of a bond. These "lone pairs" of electrons push the other atoms away, changing the molecule's shape.
Common Molecular Shapes
Here are some other common shapes molecules can have:
- Linear: Two atoms connected to a central atom, with no lone electron pairs. They form a straight line (180° angle).
- Trigonal Planar: Three atoms connected to a central atom, with no lone electron pairs. They lie flat in a triangle shape.
- Trigonal Pyramidal: Three atoms connected to a central atom, plus one lone electron pair. This makes a pyramid shape.
- Tetrahedral: Four atoms connected to a central atom, with no lone electron pairs. This shape looks like a pyramid with four triangular faces.
- Trigonal Bipyramidal: Five atoms connected to a central atom, with no lone electron pairs. This shape looks like two pyramids joined at their bases.
- Octahedral: Six atoms connected to a central atom, with no lone electron pairs. This shape has eight faces, like a diamond.
Knowing a molecule's shape helps scientists understand how it works. It's a key part of chemistry!
See also
 In Spanish: Geometría molecular para niños
In Spanish: Geometría molecular para niños
 | Precious Adams |
 | Lauren Anderson |
 | Janet Collins |

