Water (molecule) facts for kids
Water (H2O) is a very important molecule that covers about 70-75% of Earth's surface. You can find it as a liquid, as a solid (ice), and as a gas (vapor) in the air. It is the most common molecule on our planet.
At normal room temperature, water is a clear liquid. It has no taste and no smell. Many things can dissolve in water, which is why it is often called the universal solvent. Because of this, water in nature is rarely completely pure. It often has other substances mixed in, which can change its properties a little bit. However, some things do not dissolve in water at all. Water is special because it is the only common substance found naturally in all three states of matter: solid, liquid, and gas.
Pure water has no taste. Any taste you notice in water comes from other chemicals that are dissolved in it.
Liquid water freezes and turns into solid ice at a temperature of 0 degrees Celsius (32 degrees Fahrenheit or 273 Kelvin).
Contents
Water's Amazing Properties
Most liquids get bigger (expand) when they are heated. But water is different! When water is heated from 0°C to 4°C, it actually gets smaller (its volume decreases). It only starts to expand when heated above 4°C. This unusual behavior is one of water's special properties.
This special property helps fish and other water animals survive in cold places. When a lake or pond gets very cold, the water at the top cools down first. As it cools below 4°C, it becomes less dense and rises instead of sinking. This means the coldest water (0-3°C) stays near the surface, while the slightly warmer, denser water (4°C) stays at the bottom. Ice then forms on the very top. Since ice is a poor conductor of heat, it acts like a blanket, stopping the heat from escaping the water below. This keeps the water underneath from freezing, allowing aquatic animals to live through the winter.
What is Water Made Of?
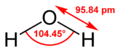
Water is a chemical substance with the formula H2O. This means one molecule of water has two hydrogen atoms connected to one oxygen atom. They are held together by strong connections called covalent bonds.
Liquid water is mostly clear, but it can look a little blue. You can see this blue color in a full bathtub or a large glacier. This color comes from how water absorbs tiny amounts of light.
Water is usually a liquid at normal temperatures and pressures. Other similar substances, like those from the oxygen family, are usually gases. Water is different because its molecules are attracted to each other through special connections called hydrogen bonds. These bonds are always breaking and reforming very quickly. Even though they are temporary, these bonds are strong enough to give water many of its unique properties, which are essential for life on Earth.
Water, Ice, and Vapor
On Earth, the liquid form of water is the most common. We usually just call it "water." The solid form of water is called ice. Ice can be hard, like ice cubes, or soft and fluffy, like snow. There are also other types of ice besides the common hexagonal ice. The gas form of water is called water vapor or steam. When you see steam or clouds, they are actually made of tiny water droplets floating in the air.
Images for kids
-
Dew drops sticking to a spider web.
-
Rain water falling from a tree. Forces like Surface tension help drops form.
-
This paper clip is floating on the water's surface. Surface tension stops it from sinking.
-
The water at Havasu Falls looks turquoise because of tiny bits of calcium carbonate dissolved in it.
See also
 In Spanish: Agua para niños
In Spanish: Agua para niños
 | Roy Wilkins |
 | John Lewis |
 | Linda Carol Brown |