Surface tension facts for kids
Surface tension is like an invisible, stretchy skin on the surface of a liquid. This "skin" is strong enough to hold up light things, like a paper clip, or even allow some insects to walk on water! It also makes water droplets form into tiny balls.
This amazing property happens because the tiny molecules inside a liquid are always pulling on each other. This pulling is called cohesion.
Surface tension is measured as a force per unit of length. Think of it as how much strength the "skin" has along a certain line. It can also be thought of as energy per unit of area, which is called surface energy. This term is more general and can apply to solids too.
In materials science, surface tension helps us understand how materials behave at their surfaces.
Contents
What Causes Surface Tension?
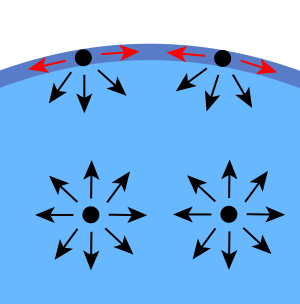
Surface tension happens because liquid molecules are attracted to each other. Imagine molecules deep inside a liquid: they are pulled equally in every direction by their neighbors. This means the total pull on them is zero.
But molecules right at the surface are different. They don't have other molecules above them. So, they are pulled mostly inwards and sideways by their neighbors. This inward pull makes the liquid surface try to shrink to the smallest possible area.
This is why liquid droplets are usually round. A sphere (a perfect ball shape) has the smallest surface area for its amount of liquid. So, the liquid pulls itself into a ball to use the least amount of "skin" possible. Think of it like a balloon trying to shrink to its smallest size.
Another way to think about it is with energy. Molecules that are touching other molecules have less energy than those that are alone. Molecules on the surface have fewer neighbors than those inside. This means surface molecules have higher energy. To lower the total energy of the liquid, it tries to have as few high-energy surface molecules as possible. This means it tries to have the smallest surface area.
Because liquids try to minimize their surface area, their surfaces become as smooth as possible. Any curve or bump would mean more surface area and higher energy. So, the surface pushes back against any bending.
Surface Tension in Everyday Life
Surface tension is all around us! Here are some cool examples, especially with water:
Water's Amazing Tricks
- A. Raindrops on a leaf: Have you ever seen raindrops form perfect beads on a waxy leaf? Water doesn't stick well to wax, but it sticks strongly to itself. Surface tension pulls the water into round drops, because a sphere has the smallest surface area for its size.
- B. Drops from a faucet: When water drips from a tap, it forms individual drops. The water hangs on, getting bigger, until surface tension can't hold it anymore. Then, it breaks off, and surface tension pulls the falling water into a sphere. Even a steady stream of water will eventually break into drops as it falls, because gravity stretches it and surface tension pinches it into spheres.
- C. Insects walking on water: Some insects, like water striders, can walk on water! Even though they are heavier than water, surface tension acts like a trampoline. Their feet make small dents in the water's surface, and the water's "skin" pushes back, holding them up.
- D. Oil and water separating: When you mix oil and water, they don't blend. This is because there's a special kind of surface tension, called "interface tension," between the two different liquids. It makes them stay apart.
- E. "Tears of wine": When you swirl wine in a glass, you might see drops and streaks forming on the sides. This happens because alcohol evaporates faster than water. The different surface tensions of water and alcohol create this interesting effect.
-
C. Water striders stay atop the liquid because of surface tension.
-
D. Lava lamp with interaction between dissimilar liquids; water and liquid wax.
How Soaps Help
Surface tension also explains why surfactants (like soap) are so useful:
- Soap bubbles: Bubbles have a huge surface area but very little mass. Pure water bubbles are unstable, but adding surfactants makes them last longer. Surfactants actually lower the surface tension of water, making it easier to create and stabilize bubbles.
- Emulsions: An emulsion is a mixture where tiny drops of one liquid are spread throughout another (like oil in water). Without a surfactant, these tiny oil drops would quickly join together into bigger blobs. But surfactants reduce the surface tension between the oil and water, allowing the tiny drops to stay separate and stable.
How We Measure Surface Tension
Since surface tension causes many different effects, there are many ways to measure it. The best method depends on the liquid and how stable its surface is.
Here are a few common methods:
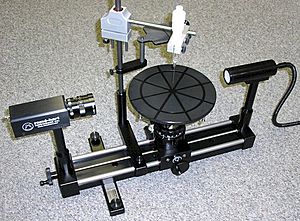
- Du Noüy Ring method: This is a classic way to measure surface tension. A small ring is pulled up from the liquid surface, and the maximum force needed to pull it away is measured.
- Wilhelmy plate method: A flat plate is dipped into the liquid, and the force needed to wet the plate is measured. This method is good for checking surface tension over a long time.
- Pendant drop method: Scientists take a picture of a hanging drop of liquid. By analyzing its shape, they can figure out the surface tension. This works even at high temperatures.
- Capillary rise method: A very thin tube (called a capillary) is placed in the liquid. The liquid will rise up the tube to a certain height because of surface tension. By measuring this height, you can calculate the surface tension.
More Effects of Surface Tension
Liquid in a Thin Tube
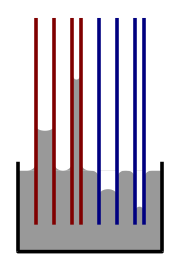
If you put a very thin tube into a liquid, something interesting happens. If the liquid sticks to the walls of the tube (like water in a glass tube), surface tension will pull the liquid up the tube. This is called capillary action. The thinner the tube, the higher the liquid will rise!
The height the liquid rises depends on:
- The liquid's surface tension.
- How much the liquid sticks to the tube (the contact angle).
- The density of the liquid.
- The radius (half the width) of the tube.
- Gravity.
If the liquid doesn't stick to the tube (like mercury in a glass tube), surface tension will actually push the liquid down in the tube, making a curved dip.
Puddles on a Surface
When you pour mercury onto a flat glass surface, it forms a puddle that has a certain thickness, not spreading out super thin. This is because mercury has very strong surface tension. It tries to flatten out to lower its overall level, but at the same time, surface tension tries to reduce the total surface area. The result is a puddle with a specific thickness.
You can see a similar effect with water on a waxy surface (like a waxed car hood). Water doesn't stick to wax, so it beads up and forms puddles with a noticeable thickness, just like mercury on glass.
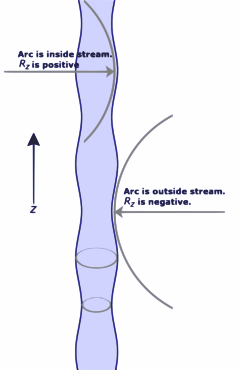
Why Streams Break into Drops
Have you ever noticed that a stream of water coming from a faucet always breaks up into individual droplets, even if it starts out smoothly? This is due to something called the Plateau–Rayleigh instability, and it's all thanks to surface tension.
Even the smoothest stream of water has tiny wobbles or disturbances. Surface tension acts on these wobbles. It makes some of them grow bigger and bigger, while others shrink. Eventually, the wobbles that grow cause the stream to pinch off and break into separate drops. This is how nature tries to reduce the surface area of the water.
Surface Tension Data
Here's a table showing the surface tension of different liquids against air. The numbers are in dynes per centimeter (dyn/cm), which is the same as milli-Newtons per meter (mN/m).
| Liquid | Temperature °C | Surface tension, γ |
|---|---|---|
| Acetic acid | 20 | 27.6 |
| Acetic acid (40.1%) + Water | 30 | 40.68 |
| Acetic acid (10.0%) + Water | 30 | 54.56 |
| Acetone | 20 | 23.7 |
| Diethyl ether | 20 | 17.0 |
| Ethanol | 20 | 22.27 |
| Ethanol (40%) + Water | 25 | 29.63 |
| Ethanol (11.1%) + Water | 25 | 46.03 |
| Glycerol | 20 | 63 |
| n-Hexane | 20 | 18.4 |
| Hydrochloric acid 17.7M aqueous solution | 20 | 65.95 |
| Isopropanol | 20 | 21.7 |
| Liquid Nitrogen | -196 | 8.85 |
| Mercury | 15 | 487 |
| Methanol | 20 | 22.6 |
| n-Octane | 20 | 21.8 |
| Sodium chloride 6.0M aqueous solution | 20 | 82.55 |
| Sucrose (55%) + water | 20 | 76.45 |
| Water | 0 | 75.64 |
| Water | 25 | 71.97 |
| Water | 50 | 67.91 |
| Water | 100 | 58.85 |
Gallery of Surface Tension Effects
Images for kids
See also
 In Spanish: Tensión superficial para niños
In Spanish: Tensión superficial para niños