Water (data page) facts for kids
Water is an amazing and essential substance for all life on Earth! This page shares some cool facts and numbers about water, helping you understand its unique characteristics. It's like a secret file with extra details about the properties of water.
Contents
The Amazing Structure of Water
Water molecules (H₂O) are made of two hydrogen atoms and one oxygen atom. These atoms are connected in a special way, forming a bent shape. The angle between the hydrogen atoms is about 104.48 degrees. This special shape helps water do many important things, like dissolving other substances.
Water's Phases and Changes
Water can exist as a solid (ice), a liquid (water), or a gas (steam).
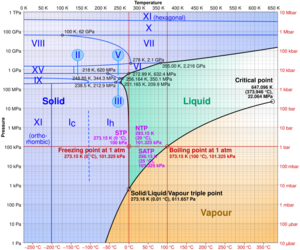
- The triple point is a special temperature and pressure. Here, water can be ice, liquid, and steam all at once! For water, this happens at 0.01°C (32.018°F) and a very low pressure.
- The critical point is another unique condition. Above 374°C (705°F) and a very high pressure, water doesn't have a clear difference between liquid and gas. It becomes a "supercritical fluid."
How Water Changes State
When ice melts into liquid water, it absorbs energy. This is called the enthalpy of fusion. When liquid water turns into steam, it also absorbs a lot of energy. This is known as the enthalpy of vaporization. These energy changes are why water is so good at storing and moving heat around the planet.
Liquid Water's Cool Features
Water has many interesting physical properties when it's in its liquid form.
Sound in Water
Did you know sound travels much faster in water than in air? In pure water at 25°C (77°F), sound zips along at about 1498 meters per second! That's about four times faster than in air.
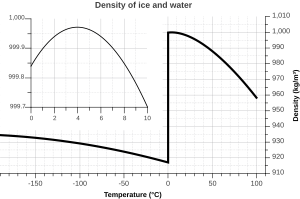
Water's Density Secrets
Water has a unique density. Most liquids get denser as they get colder. But water is densest at about 4°C (39.2°F). This is why ice floats! As water freezes into ice, it actually becomes less dense. This is super important for life in lakes and oceans. Ice forms on top, insulating the water below.
How Thick is Water? (Viscosity)
Viscosity describes how "thick" or "sticky" a liquid is. It also tells us how much it resists flowing. Water isn't very viscous, meaning it flows easily. Its viscosity changes with temperature; colder water is a bit thicker than warmer water.
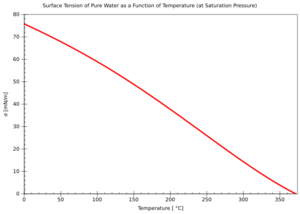
The "Skin" of Water (Surface Tension)
Water molecules at the surface are more attracted to each other than to the air above. This creates a kind of "skin" on the water, called surface tension. It's why small insects can walk on water and why water forms droplets.
Does Pure Water Conduct Electricity?
Very pure water doesn't conduct electricity very well. This is because it doesn't have many free charged particles (ions) to carry the electric current. However, if you add even a tiny bit of salt or other minerals, it becomes a much better conductor!
Ice Under Pressure
The melting point of ice isn't always 0°C (32°F). If you put ice under a lot of pressure, its melting point can actually go down. This is why ice skates can glide on ice. The pressure from the blade slightly melts the ice, creating a thin layer of water.
Water's Phase Diagram
This diagram, called a phase diagram, is like a map. It shows you what state water will be in (solid, liquid, or gas) at different temperatures and pressures. The lines on the map show where water changes from one state to another. You can see how many different types of ice exist under extreme conditions!
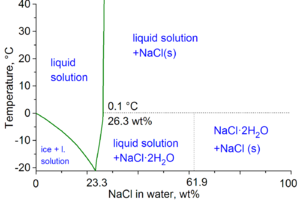
Saltwater: A Different Story
When you add salt (like NaCl) to water, it changes some of its properties. For example, saltwater freezes at a lower temperature and boils at a higher temperature than pure water. This is why people put salt on icy roads in winter! The more salt you add, the more these points change.
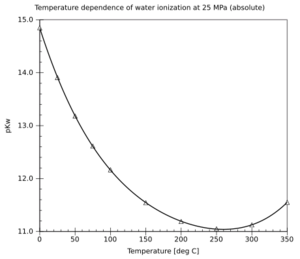
Water's Tiny Split-Up (Self-Ionization)
Even in pure water, a very small number of water molecules can split apart. They form charged particles called hydrogen ions (H⁺) and hydroxide ions (OH⁻). This process is called self-ionization. It's a fundamental chemical reaction that helps us understand pH and how acidic or basic a solution is. The amount of splitting changes with temperature.
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.
See also