- This page was last modified on 17 October 2025, at 10:18. Suggest an edit.
pH facts for kids

Lemon juice tastes sour because it has a low pH, around 2.2. This means it is quite acidic!
pH is a special way to measure how acidic or alkaline (which is also called basic) something is. Think of it like a scale that goes from 0 to 14.
If a substance has a pH less than 7, it's an acid. The lower the number, the stronger the acid. For example, battery acid has a pH of 1.
If a substance has a pH greater than 7, it's an alkali (or a base). The higher the number, the stronger the alkali. Bleach, for instance, has a pH of about 12.3.
Substances that are neither acidic nor alkaline are called neutral. Pure water is a great example, and it usually has a pH of 7.
The idea of pH was introduced by a scientist named S.P.L. Sørensen in 1909. The "p" in pH comes from a German word meaning "power" or "concentration," and the "H" stands for hydrogen ions. These tiny particles help us understand how acidic or alkaline a solution is.
Contents
How We Measure pH
A pH indicator is a special chemical that helps us see the pH of a liquid. You add a small amount of the indicator to a solution, and it changes color! The color tells you if the solution is acidic, neutral, or alkaline.
Some common indicators are phenolphthalein and methyl orange. Each one changes color at different points on the pH scale. You can even mix several indicators to make a universal indicator, which shows many different colors for different pH levels.
Another easy way to check pH is by using litmus paper. This paper has natural pH indicators on it. When you dip it into a liquid, it changes color to show you if the liquid is an acid or a base.
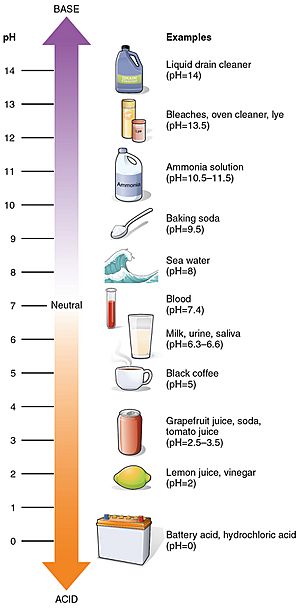
pH Values of Everyday Things
Many things you use every day have a pH value. Here are a few examples:
- Battery acid: 1.0 (very acidic!)
- Lemon juice: 2.4
- Vinegar: 3.0
- Coffee: 5.0
- Milk: 6.6
- Pure water: 7.0 (neutral)
- Blood: 7.35 - 7.45 (slightly alkaline)
- Sea water: 8.0
- Hand soap: 9.0 - 10.0
- Household ammonia: 11.5
- Bleach: 12.3 (very alkaline!)
What is Neutralisation?
When an acid and a base mix, they can cancel each other out. This process is called neutralisation. It often creates water and a salt.
You can think of it like this: H+ (from an acid) + OH− (from a base) → H2O (water)
So, an acid plus a base usually makes water!
Related pages
Images for kids
-
Test tubes containing solutions of pH 1–10 colored with an indicator
-
Lemon juice tastes sour because it contains 5% to 6% citric acid and has a pH of 2.2 (high acidity).
See also
 In Spanish: PH para niños
In Spanish: PH para niños