Titration facts for kids
Titration is a cool chemistry trick! It helps scientists figure out how much of a certain substance is in a liquid. Imagine you have a mystery drink, and you want to know how much sugar is in it. Titration is like a super-accurate measuring method to find that out.
Contents
What is Titration?
Titration is a special way to do quantitative chemical analysis. This means it's about measuring exact amounts. Scientists use it to find the unknown concentration of a substance. Concentration tells you how much of something is dissolved in a liquid.
How Does Titration Work?
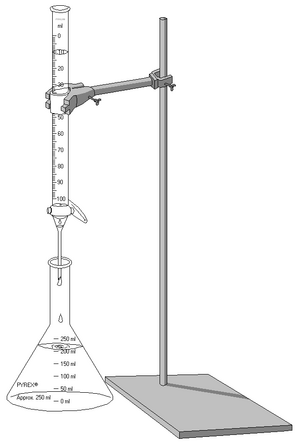
To do titration, chemists use specific tools. The two most important ones are the pipette and the burette. They also use special chemicals called indicators. These indicators change color when the reaction is finished. Common indicators include phenolphthalein or methyl orange. Sometimes, the chemical itself, like KMnO4 (potassium permanganate), can act as an indicator.
Here's how it works:
- You start with a known amount of a substance you want to test. This is called the analyte or titrand.
- Then, you slowly add another chemical, called the titrant, from a burette. You know exactly how strong (its concentration) this titrant is.
- You keep adding the titrant until the reaction is complete. This point is called the end point. You usually see the end point when the indicator changes color.
- You then note down exactly how much titrant you added.
- Because you know the strength of the titrant and how much you used, you can then calculate the unknown strength of your original substance!
Why is it Called Volumetric Analysis?
Measuring volumes (how much liquid you have) is super important in titration. That's why titration is also known as volumetric analysis. It's all about precise measurements of liquids to find out chemical secrets!
Modern Titration Methods
Today, many professionals use a special device called a pH meter. This device is much more accurate than color-changing indicators. A pH meter has an electrode that can measure how many H+ ions are in the solution.
As the titrant is added, the pH meter constantly measures the pH of the solution. When the end point is reached, there's a sudden and clear change in the measured pH. This gives a very precise result.
Images for kids
-
A burette and Erlenmeyer flask (conical flask) being used for an acid–base titration.
See also
 In Spanish: Análisis volumétrico para niños
In Spanish: Análisis volumétrico para niños
 | Charles R. Drew |
 | Benjamin Banneker |
 | Jane C. Wright |
 | Roger Arliner Young |