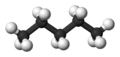Pentane facts for kids
Pentane is a type of chemical compound that is made up of carbon and hydrogen atoms. Its chemical formula is C5H12. This means each molecule of pentane has 5 carbon atoms and 12 hydrogen atoms. Pentane belongs to a group of chemicals called alkanes, which are known for being very stable.
When people say "pentane," they usually mean any of the three different forms (called isomers) that have the same chemical formula but different arrangements of atoms. These are n-pentane, isopentane, and neopentane. However, in official chemistry rules (from the IUPAC), the name pentane specifically refers to n-pentane. The other two isomers have different official names: 2-methylbutane and 2,2-dimethylpropane.
What are Pentane's Isomers?
Pentane has three different forms, or isomers. These isomers all have the same number of carbon and hydrogen atoms (C5H12), but their atoms are connected in different ways. Think of it like building blocks: you have the same number of blocks, but you can arrange them to make different shapes.
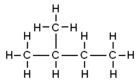
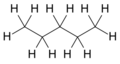
- n-pentane is a straight chain of five carbon atoms.
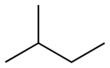
- Isopentane (also called 2-methylbutane) has a main chain of four carbon atoms with one carbon atom branching off.
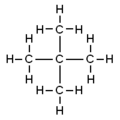
- Neopentane (also called 2,2-dimethylpropane) has a central carbon atom connected to four other carbon atoms, making it look like a cross.
These different arrangements give each isomer slightly different properties, even though they are made of the same basic parts.
| Common Name | normal pentane unbranched pentane n-pentane |
isopentane | neopentane |
| Official Name (IUPAC) | pentane | 2-methylbutane | 2,2-dimethylpropane |
| Molecular Diagram | |||
| Skeletal Diagram |
How Pentane Reacts
Like many other alkanes, pentane can burn when it mixes with oxygen. This process is called combustion. When pentane burns completely, it produces carbon dioxide gas and water vapor. This reaction releases energy, which is why pentane can be used as a fuel.
Here's the chemical equation for pentane burning:
- C5H12+8O2->5CO2+6H2O.
This equation shows that one molecule of pentane reacts with eight molecules of oxygen to create five molecules of carbon dioxide and six molecules of water.
Images for kids
See also
 In Spanish: Pentano para niños
In Spanish: Pentano para niños
 | Aaron Henry |
 | T. R. M. Howard |
 | Jesse Jackson |