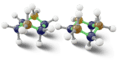Isomer facts for kids
An isomer in chemistry is like a puzzle where you have the same pieces but you put them together in a different way! Imagine you have a certain number of atoms (the tiny building blocks of everything). If you arrange these same atoms in different ways, you get different chemical compounds. These different arrangements are called isomers. Even though they have the exact same ingredients (the same molecular formula), they can look, smell, or act very differently!
There are two main types of isomers: structural isomers and stereoisomers.
Contents
Structural Isomers: Different Connections
Structural isomers are molecules that have the same number and types of atoms, but these atoms are connected in different orders. Think of it like having the same set of LEGO bricks, but you build completely different things with them.
For example, if you have a molecule with three carbon atoms, eight hydrogen atoms, and one oxygen atom (written as C₃H₈O), you can arrange them in a few different ways:
- You could have 1-propanol, where the oxygen atom is at one end.
- You could have 2-propanol, where the oxygen atom is in the middle.
- Or you could have ethyl-methyl-ether, where the oxygen atom is in between two carbon chains.
Even though all three of these have the exact same chemical formula (C₃H₈O), they are different chemicals with different properties because their atoms are connected differently.
Stereoisomers: Different Shapes in Space
Stereoisomers are a bit trickier. They have the same atoms connected in the same order, but their atoms are arranged differently in space. Imagine you have two identical gloves. They both have the same fingers and thumb, but one is a left glove and the other is a right glove – they are mirror images that can't be perfectly stacked on top of each other.
Stereoisomers can be very similar, but their slight differences in 3D shape can be very important, especially in biology. For example, your body might only be able to use one specific shape of a molecule, while the other shape might not work or could even be harmful.
Optical Isomers: Mirror Images
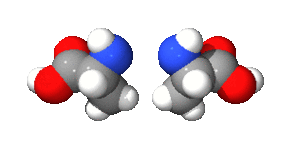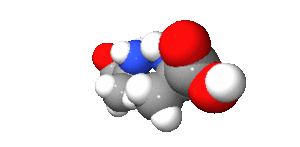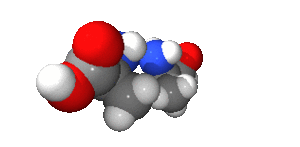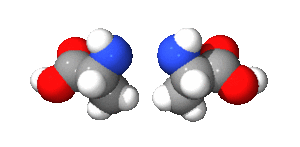
One cool type of stereoisomer is called an optical isomer (also known as enantiomers). These are molecules that are exact mirror images of each other, just like your left and right hands. You can't rotate one to make it look exactly like the other.
The image of alanine shows two optical isomers. They are identical in every way except that one is a mirror image of the other. This property is very important in medicines, as often only one of the mirror images will have the desired effect in the body.
Conformational Isomers: Twists and Turns
Another type of stereoisomer is a conformational isomer. These are molecules that can change their shape by rotating around single bonds. Think of a paper clip that you can bend and twist into different forms without breaking it. The atoms are still connected in the same order, but the molecule can take on different temporary shapes.
A good example is cyclohexane, a ring-shaped molecule. It can exist in different "conformations" like the "chair" form or the "boat" form. These forms can change into each other without breaking any chemical bonds, just by twisting.
Images for kids
See also
 In Spanish: Isomería para niños
In Spanish: Isomería para niños
 | Toni Morrison |
 | Barack Obama |
 | Martin Luther King Jr. |
 | Ralph Bunche |