Cyclohexane facts for kids
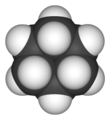
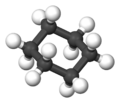
Cyclohexane is a special type of molecule called a cycloalkane. It has the formula C6H12. This means it has 6 carbon atoms linked together in a ring, and each carbon atom is also connected to two hydrogen atoms.
Cyclohexane is a clear liquid with a sweet, gasoline-like smell. It is very flammable, meaning it can easily catch fire.
Contents
What is Cyclohexane Used For?
Cyclohexane is an important chemical used to make other useful substances. It is mainly used to create adipic acid and caprolactam. These two chemicals are then used to produce nylon, which is a strong material used in many products, like clothes, ropes, and plastics.
It can also be used as a solvent, which means it can dissolve other substances. However, it does not mix with water.
Properties of Cyclohexane
Cyclohexane is a colorless liquid. It has a density of about 0.7781 grams per milliliter. This means it is lighter than water.
Melting and Boiling Points
Cyclohexane melts at 6.47 degrees Celsius. This is just above the freezing point of water. It boils at 80.74 degrees Celsius. This is a bit higher than the boiling point of water.
Safety Information
Cyclohexane is flammable and can be harmful if swallowed or if its vapors are breathed in. It can also irritate the skin. Because it is flammable, it should be kept away from heat and open flames.
Related Chemicals
Cyclohexane belongs to a group of chemicals called cycloalkanes. These are molecules with carbon atoms arranged in a ring.
- Cyclopentane: This is a similar molecule but has 5 carbon atoms in its ring.
- Cycloheptane: This molecule has 7 carbon atoms in its ring.
Other related chemicals include Cyclohexene, which has a double bond in its ring, and Benzene, which is an aromatic compound with a stable ring structure.
Images for kids
See also
 In Spanish: Ciclohexano para niños
In Spanish: Ciclohexano para niños
 | William Lucy |
 | Charles Hayes |
 | Cleveland Robinson |