Alkene facts for kids
In organic chemistry, an alkene is a special type of chemical compound. It's also called an olefin. What makes an alkene special is that it has at least one carbon atom connected to another carbon atom by a double bond. Think of it like two hands holding each other very strongly.
The simplest alkenes have just one double bond. They don't have rings or other special parts. These are hydrocarbons, meaning they are made only of hydrogen and carbon. Their general formula is CnH2n. This means if there are 'n' carbon atoms, there will be '2n' hydrogen atoms.
Even though some drawings of other chemicals called 'aromatic compounds' might look like alkenes, they are actually quite different. They are not considered alkenes.
The double bond in alkenes makes them more reactive than other similar compounds. This is because they are "unsaturated," meaning they have room to add more atoms. For example, an alkene can easily react with bromine, which makes the bromine lose its color. The names of all alkenes always end with "-ene."
Contents
What are Some Alkenes?
Here is a list of the first few alkenes. They are named based on how many carbon atoms they have:
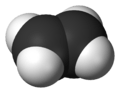
- Ethene (C2H4) - This is the simplest alkene, with two carbon atoms.
- Propene (C3H6) - Has three carbon atoms.
- Butene (C4H8) - Has four carbon atoms.
- Pentene (C5H10) - Has five carbon atoms.
- Hexene (C6H12) - Has six carbon atoms.
- Heptene (C7H14) - Has seven carbon atoms.
- Octene (C8H16) - Has eight carbon atoms.
- Nonene (C9H18) - Has nine carbon atoms.
- Decene (C10H20) - Has ten carbon atoms.
- Undecene (C11H22) - Has eleven carbon atoms.
- Dodecene (C12H24) - Has twelve carbon atoms.
- Tridecene (C13H26) - Has thirteen carbon atoms.
- Tetradecene (C14H28) - Has fourteen carbon atoms.
- Pentadecene (C15H30) - Has fifteen carbon atoms.
- Hexadecene (C16H32) - Has sixteen carbon atoms.
- Heptadecene (C17H34) - Has seventeen carbon atoms.
How Alkenes Look and Feel
The way alkenes look and feel is quite similar to alkanes, which are compounds with only single bonds. The main difference is how they react.
The physical state of an alkene (whether it's a gas, liquid, or solid) depends on how many carbon atoms it has.
- The simplest alkenes, like ethene, propene, and butene, are gases at room temperature.
- Alkenes with about five to sixteen carbon atoms are liquids.
- Alkenes with even more carbon atoms are waxy solids.
Why Alkenes Are Not Used as Fuel
Alkenes are generally not used as fuels for a couple of reasons:
- They are not very common in nature. Instead, they are often made from other hydrocarbons. These alkenes are then used to create many useful things like plastics, anti-freeze, and other important compounds.
- When alkenes burn, they often produce a smoky flame. This happens because they don't burn as completely as some other fuels. This means they release less heat energy and create more pollution.
Images for kids
-
A 3D model of ethylene, the simplest alkene.
See also
 In Spanish: Alqueno para niños
In Spanish: Alqueno para niños