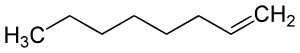
Octene facts for kids
Octene is a type of chemical compound that is part of a group called alkenes. Its chemical formula is C8H16. This means that each molecule of octene is made up of eight carbon atoms and sixteen hydrogen atoms.
What makes octene special, like all alkenes, is that it has at least one double bond between two of its carbon atoms. This double bond makes it more reactive than other similar chemicals that only have single bonds.
Contents
What is Octene?
Octene is a colorless liquid. It is not very soluble in water, meaning it doesn't mix well with it. However, it can mix with other organic liquids.
Different Kinds of Octene
There isn't just one type of octene! Because the double bond can be in different places along the chain of carbon atoms, and the atoms can be arranged in different ways, there are actually many different forms of octene. These different forms are called isomers. For example, "1-octene" means the double bond is on the first carbon atom. Other types include 2-octene, 3-octene, and 4-octene, depending on where the double bond is located.
How is Octene Used?
Octene is a very useful chemical in many industries. It is mainly used as a "building block" to create other important substances.
Making Plastics
One of the main uses for octene is in making plastics. It is often used to create a type of plastic called polyethylene. When octene is added to polyethylene during its production, it helps to make the plastic stronger and more flexible. This type of plastic is used for things like packaging films, pipes, and even some car parts.
Other Chemical Products
Octene is also used to make other chemicals. For example, it can be turned into alcohols, acids, and other organic compounds. These compounds are then used in a wide range of products, such as:
- Detergents (for cleaning)
- Lubricants (to reduce friction in machines)
- Adhesives (glues)
- Cosmetics (beauty products)
How is Octene Made?
Octene is usually produced in large industrial plants. It is often made from petroleum, which is a fossil fuel. The process involves breaking down larger hydrocarbon molecules into smaller ones, like octene, using high temperatures and special catalysts. This process is called cracking.
Safety Information
Like many chemicals, octene should be handled carefully. It is flammable, meaning it can easily catch fire. It also has a strong odor and can be irritating if it comes into contact with skin or eyes. Because of this, it is always handled by trained professionals in controlled environments.