Organic chemistry facts for kids

Organic chemistry is a big part of chemistry that studies special chemical compounds. These compounds all have one thing in common: they contain the element carbon. Carbon is super unique because it can connect with many other chemical elements and even with other carbon atoms. This means carbon can form a huge, almost endless, number of different molecules!
The word "organic" comes from the idea that all known living things, like plants, animals, and even us, are mostly made up of water and these carbon compounds. So, organic chemistry helps us understand the building blocks of life. Organic chemists often work to create, or synthesize, new organic products by mixing different chemicals in chemical reactions. Many exciting fields, like biochemistry (the chemistry of living things), microbiology (the study of tiny living things), and medicine, use ideas from organic chemistry.
Contents
What is Organic Chemistry?
Organic chemistry is all about understanding how carbon atoms connect to each other and to other atoms. Think of carbon as a super-connector! Because carbon can form so many different kinds of bonds, it creates a huge variety of molecules. These molecules are everywhere around us, from the food we eat to the clothes we wear and the medicines we take.
A Quick Look at History
The term "organic" was first used by a Swedish scientist named Jöns Jacob Berzelius in the 19th century. He used it to describe substances found only in living things. Back then, people believed in something called the "vital force theory." This theory said that you needed a special "life force" to make organic compounds.
But this idea started to change in 1828. A scientist named Friedrich Wöhler did an experiment that proved the vital force theory wrong. He managed to create urea, which is an organic compound, from ammonium cyanate, which is an inorganic (non-living) compound. This showed that organic compounds could be made in a lab, not just by living things.
Understanding Hydrocarbons
A very important part of organic chemistry is the study of hydrocarbons. These are molecules that contain only two elements: carbon and hydrogen. They often form long chains or rings.
Hydrocarbons are split into two main types:
- Aliphatic hydrocarbons do not have a special ring structure called a benzene ring.
- Aromatic hydrocarbons do contain a benzene ring.
How Organic Reactions Happen
Organic chemistry reactions happen because electrons in chemical bonds are not always shared equally. Imagine electrons as tiny magnets. Some atoms or molecules, like oxygen and nitrogen, have extra electrons and are called nucleophiles. They are attracted to positive charges. Other atoms or molecules, like H+ (a hydrogen atom with a positive charge), are called electrophiles. They are attracted to negative charges.
When an organic molecule has a positive charge, it's called a carbocation, and it acts as an electrophile. When nucleophiles and electrophiles meet, they can react and form new molecules!
Common Types of Reactions
A reaction mechanism is like a step-by-step recipe for how a chemical reaction happens. Two basic types of reactions are very important in organic chemistry: substitution and elimination reactions. Many more complex reactions use these basic steps.
Substitution Reactions
Nucleophilic substitution is when one atom or group of atoms breaks off from an organic molecule and is replaced by another.
- If the old group leaves and the new group joins at the same time, it's called an SN2 reaction.
- If the old group leaves first, creating a carbocation (a positively charged carbon atom), and then the new group joins, it's called an SN1 reaction.
Elimination Reactions
Elimination reactions happen when two groups are removed from an organic molecule, often by a strong acid. When these groups leave, the molecule forms a double bond. Usually, one of the groups removed is a nucleophile, and the other is a hydrogen atom.
- If both groups are pulled off at the same time, it's called an E2 reaction.
- If one group is pulled off first, forming a carbocation, before the second group is removed, it's called an E1 reaction.
What is Stereochemistry?
Stereochemistry is the study of how atoms are arranged in molecules in three-dimensional space. It's like looking at the shape of a molecule and how its parts are positioned relative to each other. Molecules that have the same chemical formula but are arranged differently in space are called isomers. A famous chemist named Louis Pasteur was one of the first to study stereochemistry.
A key idea in stereochemistry is chirality. Simply put, chirality looks at the symmetry of chemical molecules. If an object cannot be perfectly placed on top of its mirror image (like your left and right hands), it is called a chiral object. If it can be perfectly placed on its mirror image, it's called achiral.
Using Spectroscopy to Study Molecules
Spectroscopy is a way to study how light energy interacts with matter. For example, we see colors because organic and inorganic compounds absorb different amounts of light energy. When a plant does photosynthesis, it captures energy from the sun, which is an interaction between energy and organic compounds.
Spectroscopy is a powerful tool that helps scientists identify different organic molecules, even in unknown substances. There are many types of spectroscopy, but two very important ones for organic chemistry are infrared spectroscopy and nuclear magnetic resonance spectroscopy.
- Journal of Organic Chemistry (subscription required) (Table of Contents)
- Organic Letters (Pubs.ACS.org, Table of Contents)
Images for kids
-
Friedrich Wöhler, the scientist who showed that organic compounds could be made in a lab.
-
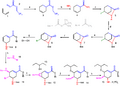
This diagram shows the many steps involved in making a medicine called oseltamivir (also known as Tamiflu). This process was designed by chemist E.J. Corey.
See also
 In Spanish: Química orgánica para niños
In Spanish: Química orgánica para niños
 | Percy Lavon Julian |
 | Katherine Johnson |
 | George Washington Carver |
 | Annie Easley |