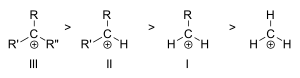Carbocation facts for kids
A carbocation is a special kind of ion in chemistry. It's an atom that has a positive charge on a carbon atom. Think of it like a carbon atom that's missing some electrons, making it positively charged.
Normally, carbon atoms like to have eight electrons in their outer shell to be stable. But a carbocation only has six electrons. This makes them very reactive and eager to find more electrons to become stable and neutral again. Even though you might think they'd have a certain shape, they actually tend to be flat, like a triangle.
Contents
What are Carbocations?
In simple terms, a carbocation is any carbon atom that has a positive charge. Sometimes, chemists use more specific names like carbenium ion for a carbon with three bonds and a positive charge, or carbonium ion for a carbon with five or six bonds and a positive charge. Most of the time, when people talk about carbocations, they are usually referring to the carbenium ion type.
How Were Carbocations Discovered?
Scientists have known about these charged carbon atoms for a long time.
- In 1891, a scientist named G. Merling found a new substance that was crystalline and could dissolve in water. Later, other scientists showed it was a special kind of carbocation called tropylium bromide. This ion is special because it's considered "aromatic," which means it's extra stable due to its electron arrangement.
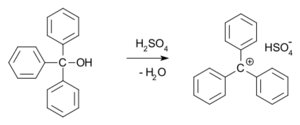
- In 1902, two scientists, Norris and Kehrman, noticed that a colorless chemical called triphenylmethanol turned bright yellow when mixed with strong sulfuric acid. Another scientist, Adolf von Baeyer, realized that these color changes were happening because new, salt-like compounds were forming with positively charged carbon atoms. He called this color change halochromy.
Carbocations are important because they are involved in many organic chemistry reactions. This idea was first suggested in 1899 by Julius Stieglitz and later developed by Hans Meerwein. For a long time, some scientific journals didn't want to publish articles that even mentioned carbocations because they were a new and debated idea!
It wasn't until 1958 that scientists could actually "see" a stable carbocation using a special technique called NMR. This helped confirm that carbocations really exist and are important in chemical reactions. In 1962, George Andrew Olah directly observed the tert-butyl carbocation, which was a big step forward.
Understanding Carbocation Properties
In chemistry, carbocations are often the target for other chemicals called nucleophiles, which are attracted to positive charges. Think of it like a magnet attracting something.
We classify carbocations based on how many other carbon atoms are directly attached to the positively charged carbon:
- Primary carbocations have one or zero other carbon atoms attached.
- Secondary carbocations have two other carbon atoms attached.
- Tertiary carbocations have three other carbon atoms attached.
The more carbon atoms attached to the positively charged carbon, the more stable the carbocation is. So, tertiary carbocations are the most stable, and primary carbocations are very unstable. This is because the nearby carbon atoms help share and spread out the positive charge, making the carbocation more comfortable.
Because primary carbocations are so unstable, many common chemical reactions that involve carbocations usually won't happen if a primary one would form. However, there's an exception: if there's a double bond next to the charged carbon, like in "allyl" or "benzyl" carbocations, they can be more stable.
Carbocations can also change their structure very quickly, rearranging themselves to become more stable. This can make it tricky to create specific chemicals because the carbocation might rearrange before it reacts the way you want it to.
Some carbocations are called "non-classical ions" because they have a slightly different way of bonding, where the positive charge is spread out over more than two atoms. This was a big topic of discussion among chemists for a long time, and George Andrew Olah helped solve the mystery of these non-classical ions.
Images for kids
See also
 In Spanish: Carbocatión para niños
In Spanish: Carbocatión para niños
 | Delilah Pierce |
 | Gordon Parks |
 | Augusta Savage |
 | Charles Ethan Porter |