Nucleophile facts for kids
A nucleophile is like a "giver" in chemistry. It's a chemical particle (a molecule or an ion) that has a spare pair of electrons. It "donates" or gives these electrons to another chemical particle called an electrophile to create a new connection, or chemical bond, in a reaction. This spare pair of electrons is called a "lone pair." Because nucleophiles give away electrons, they are also known as Lewis bases.
The word "nucleophilic" means "nucleus-loving." It describes how much a nucleophile is attracted to the center (nucleus) of another atom. "Nucleophilicity," or "nucleophile strength," tells us how good a substance is at being a nucleophile. It helps us compare how strongly different atoms are attracted to a nucleus.
When nucleophiles react with liquids like alcohols or water, these reactions are called "solvolysis." Nucleophiles are often involved in reactions where one part of a molecule is swapped for another. In these reactions, a nucleophile is drawn to a part of another molecule that has a positive electrical charge.
Contents
History of Nucleophiles
The terms nucleophile and electrophile were first used by a scientist named Christopher Kelk Ingold in 1929. Before that, in 1925, another scientist, A. J. Lapworth, had used different terms: cationoid and anionoid. Ingold's new words helped chemists better understand how these particles behave.
The word "nucleophile" comes from "nucleus" (the center of an atom) and the Greek word philos, which means "love." So, a nucleophile "loves" the nucleus.
How Nucleophiles Behave
Think of it this way:
- Basicity: If you look at chemicals across a row in the periodic table, the more "basic" an ion is (meaning it's good at accepting a proton), the better it usually is as a nucleophile.
- Polarizability: When you look down a group in the periodic table, something called "polarizability" becomes more important. This means how easy it is to stretch or distort the electron cloud around an atom or molecule. The easier it is to distort, the more easily it will react as a nucleophile. For example, the iodide ion (I−) is bigger and more easily distorted than the fluoride ion (F−), so iodide is a stronger nucleophile.
Different Kinds of Nucleophiles
Many different types of chemical particles can act as nucleophiles. They usually have a negative charge or a lone pair of electrons they can share.
For example, anions like the chloride ion (Cl−) are nucleophiles. Also, molecules like NH3 (ammonia), which has a lone pair of electrons, can be nucleophiles.
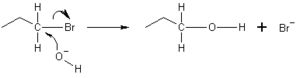
Look at the picture below. The oxygen atom in the hydroxide ion (OH−) gives an electron pair to the carbon atom in the bromopropane molecule. This causes the bond between carbon and bromine to break, and the bromine atom leaves as a bromide ion (Br−).
This type of reaction is called an SN2 reaction. The hydroxide ion attacks the carbon atom from the opposite side of where the bromine is. Because of this "backside attack," the molecule's shape can flip, like an umbrella turning inside out in the wind.
An ambident nucleophile is a special kind of nucleophile that can attack from two or more different places. This can lead to different products being formed in the same reaction. For example, the thiocyanate ion (SCN−) can attack using either its sulfur atom or its nitrogen atom. This often results in a mixture of two different products.
Carbon Nucleophiles
Carbon atoms can act as nucleophiles, especially when they are part of certain chemical compounds. Some important carbon nucleophiles are found in reactions like the Grignard reaction. These include special carbon-metal compounds and negatively charged particles of certain alkynes.
Enols are also carbon nucleophiles. They are often used in reactions that build larger molecules, like the Claisen condensation and the aldol condensation.
Oxygen Nucleophiles
Oxygen atoms are very common nucleophiles. Examples include:
- Water (H2O)
- The hydroxide anion (OH−)
- Alcohols
- Alkoxide anions
- Hydrogen peroxide
- Carboxylate anions
Sulfur Nucleophiles
Sulfur atoms are excellent nucleophiles. This is because sulfur atoms are quite large, which means their electron clouds can be easily stretched (they are very polarizable). Also, their lone pairs of electrons are easy to reach for reactions.
Common sulfur nucleophiles include:
- Hydrogen sulfide (H2S) and its salts
- Thiols (RSH)
- Thiolate anions (RS−)
Nitrogen Nucleophiles
Nitrogen atoms can also act as nucleophiles because they often have a lone pair of electrons. Some examples of nitrogen nucleophiles are:
Related pages
See also
In Spanish: Nucleófilo para niños
 | Precious Adams |
 | Lauren Anderson |
 | Janet Collins |