Thiol facts for kids
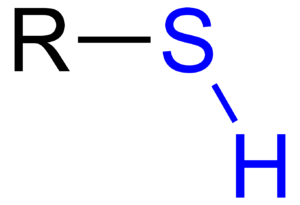
A thiol is a special type of molecule that has a specific part called an R–SH group. You can think of thiols as being very similar to alcohols, but with one key difference: instead of an oxygen atom, they have a sulfur atom in that special spot. This small change makes a big difference in how they behave!
Thiols often have a very strong and unpleasant smell. Because of this, they are added to things like natural gas so that people can easily smell if there is a leak. This is important because natural gas, which is mostly methane, doesn't have a smell on its own but can be very dangerous if it builds up.
Thiols are quite acidic, even more so than alcohols. They can also easily react with other chemicals. One of the natural amino acids, cysteine, has a thiol group in its structure, which is important for how proteins work in your body.
Contents
What Are Thiols?
Thiols are organic compounds, meaning they are built around carbon atoms. The most important part of a thiol is its "thiol group," which is made of a sulfur atom connected to a hydrogen atom (written as -SH). This group is attached to another part of the molecule, usually a chain of carbon atoms, which is often shown as 'R'. So, the general formula for a thiol is R-SH.
How Are Thiols Different from Alcohols?
You might notice that the R-SH group looks a lot like the R-OH group found in alcohols. The main difference is just one atom: alcohols have an oxygen atom (O) in their special group, while thiols have a sulfur atom (S). Even though oxygen and sulfur are in the same column on the periodic table and share some similarities, sulfur atoms are larger and behave a bit differently. This difference is why thiols have their unique properties, like their strong smell.
Why Do Thiols Smell So Bad?
Many thiols are known for their very strong and often unpleasant smells. For example, the smell of skunk spray comes from thiols! This strong smell is actually very useful. Natural gas, which we use for heating and cooking, has no smell. If there's a leak, you wouldn't know it. So, tiny amounts of a thiol called methanethiol are added to natural gas. This way, if there's a leak, you can smell it right away and stay safe.
How Thiols React
Thiols are quite reactive. They can easily undergo a process called oxidation, where they lose electrons. This is similar to how iron rusts when it oxidizes. When thiols oxidize, they can form new bonds, often connecting two thiol molecules together to create a "disulfide bond" (R-S-S-R).
Thiols are also more acidic than alcohols. This means they can more easily give away their hydrogen atom (the 'H' in -SH). When a thiol loses its hydrogen, it becomes a negatively charged particle that can react with other molecules in interesting ways.
Thiols in Your Body
Thiols are not just found in smelly gases; they are also very important in living things! One of the 20 common amino acids, which are the building blocks of proteins, is called cysteine. Cysteine has a thiol group in its structure. This thiol group is crucial for how proteins fold into their correct shapes and how they work. For example, disulfide bonds (formed from two thiol groups) help hold many proteins together, like the ones that make your hair strong.
Images for kids
See also
 In Spanish: Tiol para niños
In Spanish: Tiol para niños
 | Emma Amos |
 | Edward Mitchell Bannister |
 | Larry D. Alexander |
 | Ernie Barnes |