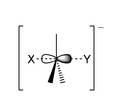SN2 reaction facts for kids
The SN2 reaction is a special type of chemical reaction in organic chemistry. It's also called bimolecular nucleophilic substitution. Imagine a tiny chemical dance where one group of atoms (the "nucleophile") replaces another group (the "leaving group") on a molecule. This happens all in one smooth step!
The "S" stands for substitution (one group replaces another). The "N" stands for nucleophilic (the attacking group is a nucleophile, which loves positive charges). And the "2" means that two different molecules are involved in the most important part of the reaction. This is why it's called "bimolecular" – "bi" means two!
Contents
How the SN2 Reaction Works
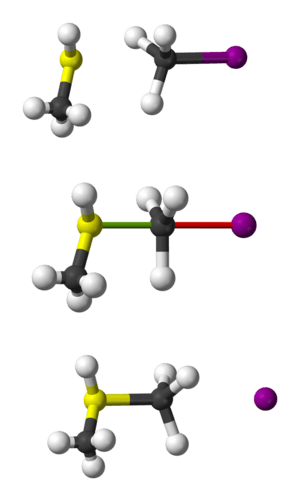
This reaction usually happens at a carbon atom that is connected to four other atoms (called an sp3 carbon). One of these atoms is a "leaving group," which is ready to leave. Often, this leaving group is a halide atom, like chlorine or bromine.
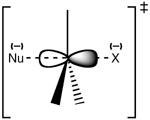
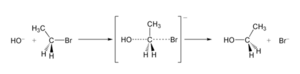
The nucleophile attacks the carbon atom from the exact opposite side of where the leaving group is attached. Think of it like a tiny chemical "backdoor" attack! As the nucleophile forms a new bond with the carbon, the leaving group is pushed away from the other side. This all happens at the same time.
In the middle of this process, there's a very short-lived "transition state." In this state, the carbon atom is temporarily connected to five things – the nucleophile, the leaving group, and the three other atoms it was already bonded to. It's like a fleeting moment where the carbon is juggling too many balls!
Walden Inversion: A Flip in Shape
If the molecule has a special 3D shape (it's "chiral"), the SN2 reaction can cause its shape to flip completely. This is like turning an umbrella inside out! This flip is called the Walden inversion.
For example, when a hydroxide ion (OH−) attacks a molecule called bromoethane, it forms ethanol. The bromide atom is the leaving group that gets pushed out.
Why Some Molecules Don't React
The SN2 reaction works best when the "backdoor" to the carbon atom is clear and not blocked by other atoms. If there are too many bulky atoms around the carbon, it's like trying to get through a crowded doorway – the nucleophile can't get in! This is called "steric hindrance."
Because of this, SN2 reactions happen most easily with simple carbon atoms (called "primary" carbons). If the carbon is very crowded (like a "tertiary" carbon), the SN2 reaction usually won't happen. Instead, a different type of reaction called an SN1 reaction might take place.
What Affects the SN2 Reaction Speed?
Four main things can change how fast an SN2 reaction happens:
- The Molecule Being Attacked (Substrate): The shape of the molecule being attacked is super important. The nucleophile needs to attack from the back. So, if the back of the molecule is clear and not blocked, the reaction will be faster. Small molecules like "methyl" and "primary" substrates react the fastest. "Secondary" substrates are a bit slower. "Tertiary" substrates are too crowded and usually don't do SN2 reactions at all.
- The Attacking Group (Nucleophile): Just like the substrate, the nucleophile's size matters. A smaller, less crowded nucleophile can attack more easily and react faster. For example, the methoxide ion is a strong nucleophile because it's small. Also, nucleophiles with a stronger negative charge or less electronegativity (meaning they hold onto their electrons less tightly) are usually better attackers.
- The Liquid It's In (Solvent): The type of liquid (solvent) where the reaction happens can affect how easily the nucleophile can attack. Some solvents, called "polar aprotic solvents" (like tetrahydrofuran), are good for SN2 reactions because they don't get in the way of the nucleophile. Other solvents, called "polar protic solvents," can surround the nucleophile and make it harder for it to attack the carbon.
- The Group That Leaves (Leaving Group): A good leaving group is one that is stable once it leaves the molecule. The more stable the leaving group, the faster the reaction. Think of it like someone who is happy to leave a party – they'll go quickly! Halides (except fluoride) and tosylate are examples of good leaving groups. But groups like HO- (hydroxide) are not good leaving groups.
How Fast Do SN2 Reactions Happen?
The speed of an SN2 reaction depends on how much of the attacking group (nucleophile) and how much of the molecule being attacked (substrate) are present. If you have more of either, the reaction will go faster. This is called a "second-order" reaction.
We can write this as a simple equation: `r = k[RX][Nu−]` Here, `r` is the reaction rate, `k` is a constant, `[RX]` is the amount of the substrate, and `[Nu−]` is the amount of the nucleophile.
This is a key difference from the SN1 reaction. In an SN1 reaction, the speed only depends on the amount of the substrate. But in an SN2 reaction, both the substrate and the nucleophile affect the speed because they both participate in the main step of the reaction.
SN2 reactions are most common with primary or secondary alkyl halides (molecules with a halogen and a simple carbon chain) when they are in a "polar aprotic solvent." They almost never happen with tertiary alkyl halides because of all the crowding around the carbon atom.
When Other Reactions Compete
Sometimes, another type of reaction called "E2 elimination" can happen at the same time as an SN2 reaction. In E2 elimination, the attacking group acts like a "base" instead of a "nucleophile." This means it removes a small part of the molecule (a proton) instead of replacing a leaving group. This leads to the formation of a different type of molecule called an alkene.
For example, in a special experiment in a gas (not a liquid), if you react a sulfonate with ethyl bromide, you mostly get the SN2 product. But if you use a more crowded molecule like isobutyl bromide, the E2 elimination reaction becomes more common because the SN2 attack is blocked.
Roundabout Mechanism: A New Idea
In 2008, scientists discovered something interesting about SN2 reactions in a gas. They found that sometimes, when chloride ions react with methyl iodide, the methyl group might spin all the way around the iodine atom before the leaving group is pushed off. This "roundabout mechanism" is a new way to think about how these reactions can happen!
Images for kids
See also
- Substitution reaction
- SN1 reaction
 In Spanish: Reacción SN2 para niños
In Spanish: Reacción SN2 para niños
 | William M. Jackson |
 | Juan E. Gilbert |
 | Neil deGrasse Tyson |