- This page was last modified on 17 October 2025, at 10:18. Suggest an edit.
SN1 reaction facts for kids
This article is about SN1 (disambiguation). For other uses, see SN1 reaction (disambiguation).
The SN1 reaction is a special type of substitution reaction in organic chemistry. Think of it like swapping one part of a molecule for another. "SN" stands for nucleophilic substitution, which means a "nucleophile" (a molecule that likes positive charges) does the swapping. The "1" tells us that the slowest step in the reaction, which controls how fast everything happens, only involves one main molecule.
This reaction creates a temporary, charged molecule called a carbocation in the middle of the process. SN1 reactions often happen with certain types of carbon chains called alkyl halides or alcohols. A scientist named Christopher Ingold first described how this reaction works in 1940.
Contents
How the SN1 Reaction Works
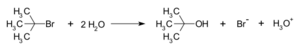
Let's look at an example: when tert-butyl bromide reacts with water to make tert-butyl alcohol. This SN1 reaction happens in three main steps:
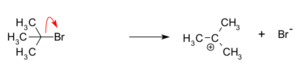
Step 1: Breaking Apart
First, the original molecule breaks apart. A "leaving group" (like a bromide ion) separates from the carbon atom. This creates a tert-butyl carbocation, which is a carbon atom with a positive charge. This step is the slowest part of the whole reaction and can even go backwards.
Step 2: New Connection Forms
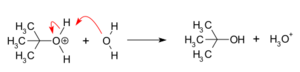
Next, the carbocation quickly reacts with a nucleophile. In our example, water acts as the nucleophile. It attacks the positively charged carbocation. If the nucleophile is a neutral molecule, like water, it forms a new, temporary molecule called an oxonium ion. This step happens very fast.
Step 3: Finishing the Product
Finally, a proton (a hydrogen atom without its electron) is removed from the new molecule formed in Step 2. Water, acting as a base, helps remove this proton. This forms the final alcohol product and a hydronium ion. This step is also very fast.
Because the first step is the slowest, it's like the "bottleneck" of the reaction. This is why chemists call it an SN1 reaction – only one molecule is involved in that crucial slow step.
When SN1 Reactions Happen
Sometimes, a molecule can react in different ways, like using an SN1 or an SN2 mechanism. The SN1 reaction usually wins when the central carbon atom is surrounded by large, "bulky" groups. These bulky groups make it hard for the SN2 reaction to happen.
Also, these bulky groups help the carbocation form more easily. The carbocation itself becomes more stable when it has these groups around it. This means the SN1 reaction is very common for carbon atoms that are connected to three other carbon groups (called "tertiary" carbons). It can also happen with "secondary" carbons (connected to two other carbon groups) if the nucleophile is not very strong.
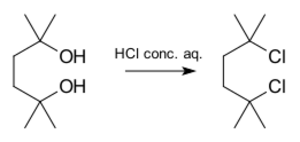
Here's an example of an SN1 reaction making 2,5-dichloro-2,5-dimethylhexane from a related molecule using strong hydrochloric acid:
How the Shape of Molecules Changes
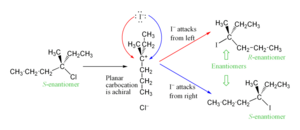
The temporary carbocation formed in an SN1 reaction has a flat, triangle-like shape. Because it's flat, the nucleophile can attack it from two different sides. If both sides are equally easy to attack, you end up with a mix of two different versions of the product. These versions are mirror images of each other, like your left and right hands. This mix is called a racemic mixture.
Look at this example: when S-3-chloro-3-methylhexane reacts with an iodide ion, it makes a racemic mix of 3-iodo-3-methylhexane.
Sometimes, one mirror image might be slightly more common. This happens if the leaving group stays close to the carbocation for a short time, blocking one side. This is different from the SN2 reaction, which always flips the molecule's shape completely.
Other Reactions That Can Happen
Sometimes, other reactions can happen at the same time as an SN1 reaction. These are called "side reactions." Two common ones are elimination reactions and carbocation rearrangement.
- Elimination reactions: If the reaction is done at warm or hot temperatures, an E1 elimination reaction might happen instead. This creates a different type of molecule called an alkene. Even at lower temperatures, some alkene might still form. If you use a very strong base as a nucleophile, an E2 elimination can also occur, especially if it's heated.
- Carbocation rearrangement: The temporary carbocation might rearrange itself into a more stable form. If this happens, the final product will be different from what you expected from a simple substitution.
How Solvents Affect the Reaction
The type of solvent (the liquid where the reaction happens) can change how fast an SN1 reaction occurs. Since the SN1 reaction creates an unstable carbocation, anything that helps stabilize this carbocation will speed up the reaction.
The best solvents for SN1 reactions are usually "polar" and "protic."
- Polar solvents are good at dissolving charged particles, which helps stabilize the temporary charged molecules.
- Protic solvents have hydrogen atoms that can form special bonds (hydrogen bonds) with the leaving group, helping it separate from the main molecule.
Common polar protic solvents include water and alcohols. These solvents can also sometimes act as the nucleophile themselves!
|
See also
 In Spanish: Reacción SN1 para niños
In Spanish: Reacción SN1 para niños