Rearrangement reaction facts for kids
A rearrangement reaction is a special type of organic reaction. In these reactions, the atoms inside a molecule move around. Imagine a molecule as a building made of carbon atoms. In a rearrangement, the "rooms" (or parts) of the building change places. This creates a new molecule that has the same atoms but in a different arrangement. It's like having the same LEGO bricks but building something new with them!
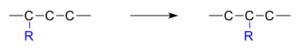
Often, a small group of atoms, called a "substituent," moves from one atom to another within the same molecule. Look at the picture below. The group "R" moves from carbon atom 1 to carbon atom 2.
Sometimes, these rearrangements can happen between different molecules too.
Chemists often draw diagrams with arrows to show how tiny particles called electrons move during these reactions. Many organic chemistry books use these diagrams. However, these diagrams don't always show the full story of how the reaction really happens. For example, when a group of atoms called an "alkyl group" moves, it often slides smoothly along a chemical bond. It's not always about bonds breaking and forming in a simple way.
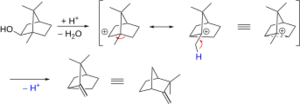
One famous example of this is the Wagner-Meerwein rearrangement.
In some reactions, like pericyclic reactions, the way electrons are arranged in the molecule's "orbitals" is very important. These reactions are complex and can't be explained by simple electron movements. However, diagrams with curved arrows can still help us understand the final result.
Three important types of rearrangement reactions are 1,2-rearrangements, pericyclic reactions, and olefin metathesis.
1,2-Rearrangements
A 1,2-rearrangement is an organic reaction where a group of atoms moves from one atom to a nearby, or "adjacent," atom in a chemical compound. The "1,2" means the group moves between two atoms that are right next to each other. Sometimes, groups can move over longer distances too, but 1,2 shifts are very common.
Here are some examples:
- The Wagner-Meerwein rearrangement:
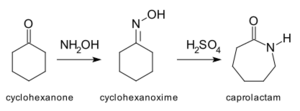
- The Beckmann rearrangement:
Pericyclic Reactions
A pericyclic reaction is a type of reaction where many carbon-carbon bonds are made and broken at the same time. During the reaction, the molecule goes through a special shape called a "transition state." This shape looks like a ring or a cycle. The reaction happens in a "concerted" way, meaning all the bond changes happen together, not one after another.
Examples of pericyclic reactions include:
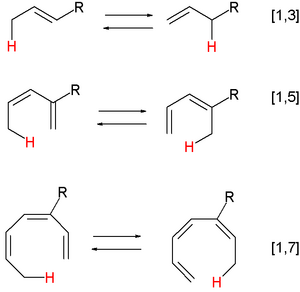
- Hydride shifts:
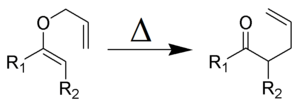
- The Claisen rearrangement:
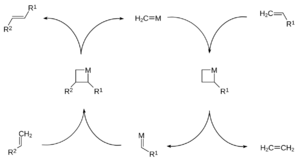
Olefin Metathesis
Olefin metathesis is a special reaction where parts of two alkene molecules are swapped. Think of it like two cars exchanging their front ends! This reaction uses a special helper called a "catalyst," which is often a transition metal carbene complex. The catalyst helps the reaction happen faster without being used up itself.
See also
 In Spanish: Reacción de transposición para niños
In Spanish: Reacción de transposición para niños
 | May Edward Chinn |
 | Rebecca Cole |
 | Alexa Canady |
 | Dorothy Lavinia Brown |