Substitution reaction facts for kids
In chemistry, a substitution reaction is a type of chemical reaction where one atom or a group of atoms in a molecule is replaced by a different atom or group. Think of it like swapping out a part of a toy for a new one! The original part leaves, and a new part takes its place.
These reactions are very common and important in many areas of chemistry, especially in organic chemistry, which is the study of molecules that contain carbon.
Contents
What is a Substitution Reaction?
A substitution reaction involves a "swap." Imagine a molecule as a small building made of different blocks (atoms). In a substitution reaction, one specific block is removed and a different block is put in its place. The rest of the building stays the same.
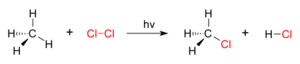
For example, if you have a molecule of methane (which is one carbon atom connected to four hydrogen atoms), a substitution reaction could happen where one of the hydrogen atoms is replaced by a chlorine atom. This would create a new molecule called chloromethane.
Types of Substitution Reactions
There are many ways substitution reactions can happen. In organic chemistry, some of the main types are:
Nucleophilic Substitution
This type of reaction involves a "nucleophile." A nucleophile is a particle that is attracted to positive charges. It has extra electrons it wants to share. In a nucleophilic substitution, this nucleophile attacks a part of a molecule that is slightly positive and replaces another group that leaves.
Electrophilic Substitution
This type involves an "electrophile." An electrophile is a particle that is attracted to negative charges. It is "electron-loving" and wants to gain electrons. In an electrophilic substitution, the electrophile attacks a part of a molecule that has a lot of electrons and replaces another group.
Other Ways Reactions Happen
Substitution reactions don't always need nucleophiles or electrophiles. Sometimes, they can happen with the help of light. These are called photochemical reactions. For example, light can help add halogen atoms (like chlorine or bromine) to molecules.
Another way is by using free radicals. Free radicals are atoms or molecules with an unpaired electron, which makes them very reactive. They can cause substitution reactions by taking an atom from one molecule and leaving another atom to take its place.
Making the Reaction Work
For a substitution reaction to happen correctly, chemists need to choose the right conditions. This includes picking the correct solvent (the liquid where the reaction takes place) and the right temperature. If the conditions aren't right, a different type of reaction, like an elimination reaction (where atoms are removed from a molecule without being replaced), might happen instead.
What is a Substituted Molecule?
The molecule that is formed after a substitution reaction is often called a "substituted molecule." For example, if you start with benzene (a ring-shaped molecule) and replace one of its hydrogen atoms with an -OH group, you get phenol. So, phenol can be called a "substituted benzene." It's like saying you have a "modified" or "changed" version of the original molecule.
See also
In Spanish: Reacción de sustitución para niños
 | Selma Burke |
 | Pauline Powell Burns |
 | Frederick J. Brown |
 | Robert Blackburn |