Phenol facts for kids
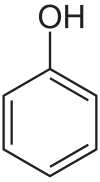
Phenol is a special type of chemical compound. It's a white solid that looks a bit like crystals. Its chemical formula is C6H5OH. This means it has a group called an "OH group" (which is short for hydroxyl group) attached to a ring of carbon atoms called a benzene ring. Even though it has an OH group like alcohols, phenol is quite acidic. This is because of how its atoms are arranged. Phenol is made from petroleum, which is a fossil fuel. It's a very important chemical because it's used to make many other useful products, like detergents (for cleaning) and herbicides (chemicals that kill weeds).
Contents
What is Phenol Like?
Why is Phenol Acidic?
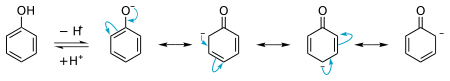
Phenol is more acidic than regular alcohols. But it's not as acidic as other compounds like carboxylic acids. Scientists measure how acidic something is using a number called pKa. Phenol has a pKa of about 10. For most alcohols, this number is usually around 15. The reason phenol is more acidic is because of its special structure. When phenol loses a hydrogen atom and becomes an anion (a negatively charged molecule), it becomes very stable. This stability makes it easier for phenol to act as an acid.
How Does Phenol React?
Phenol reacts very easily with other chemicals. These reactions are called electrophilic aromatic substitution reactions. This happens because a pair of electrons on the oxygen atom in phenol can easily move into the benzene ring. This makes the ring ready to share electrons with other molecules. Because these electrons are so active, phenol sometimes reacts more times than expected.
History of Phenol
Phenol was first found in 1834. It was discovered when scientists were studying what happens when coal burns. One of its first important uses was to kill germs. It was used to keep things clean during surgery. Today, phenol is still used in some medicines that help with pain, called analgesics.
Images for kids
See also
 In Spanish: Fenol para niños
In Spanish: Fenol para niños