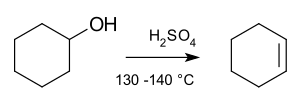Elimination reaction facts for kids
In the world of organic chemistry, an elimination reaction is a special kind of chemical change. It happens when a small part, like some atoms or a group of atoms, is removed from a larger molecule. When this happens, the molecule often forms a new double or triple bond. Think of it like taking out a few bricks from a wall to make a new opening!
Contents
How Elimination Reactions Work
There are two main ways these reactions can happen. Scientists call them E1 and E2. These names sound a bit technical, but they just describe the steps involved.
E1 Reactions: Two Steps to a New Bond
The E1 reaction happens in two separate steps.
- Step 1: Leaving Group Goes Away
First, a part of the molecule called a "leaving group" breaks off on its own. This leaving group is like a guest who decides to leave the party. When it goes, it leaves behind a special, unstable part of the molecule called a carbocation.
- Step 2: New Bond Forms
Next, a base (another chemical substance) comes along. This base takes away another small part, usually a hydrogen atom, from the molecule. As this happens, the molecule quickly rearranges itself to form a new double bond. It's like the molecule quickly fills the empty space left by the leaving group by creating a strong new connection.
E2 Reactions: One Step, All at Once
The E2 reaction is much faster because it happens all in one step.
- Simultaneous Action
In an E2 reaction, the base doesn't wait for the leaving group to go first. Instead, the base starts to take away one part (like a hydrogen atom) at the exact same time that the leaving group is breaking away. It's like two things happening at once! As these two parts leave, the new double bond forms right away. This makes the E2 reaction very efficient and quick.
A Common Example: Alcohols
A common example of an elimination reaction involves alcohols. Alcohols are molecules that contain an -OH group.
- Removing Water
If you mix an alcohol with a strong acid, something interesting happens. The acid helps to remove a molecule of water (H2O) from the alcohol. When the water molecule is eliminated, the alcohol molecule changes and forms a new double bond. This is how you can turn an alcohol into a molecule with a double bond, which is called an alkene.
Images for kids
See also
 In Spanish: Reacción de eliminación para niños
In Spanish: Reacción de eliminación para niños
 | Aaron Henry |
 | T. R. M. Howard |
 | Jesse Jackson |