Leaving group facts for kids
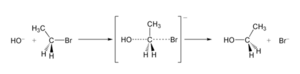
A leaving group is a part of a molecule that breaks away during a chemical reaction. Imagine a molecule as a team of atoms. In some reactions, one atom or a small group of atoms decides to leave the team. This atom or group is called the leaving group.
When a leaving group leaves, it often takes a pair of tiny particles called electrons with it.
Contents
What Makes a Good Leaving Group?
Some leaving groups are "better" than others. A good leaving group is stable and happy when it leaves the molecule and takes electrons with it. Think of it like someone who is happy to leave a party and go home.
For example, atoms from the halide family, like chlorine (Cl), bromine (Br), and iodine (I), are usually good leaving groups. This is because they are very stable when they gain an extra electron.
How Leaving Groups Affect Reactions
The type of leaving group can change how a chemical reaction happens. Different leaving groups can make certain reactions easier or harder.
For example, in a type of reaction called an SN1 reaction, it's harder for a molecule to react if it has a "bad" leaving group. But for another type of reaction, an SN2 reaction, a "bad" leaving group can actually make the reaction easier. This shows how important the leaving group is in chemistry!
Images for kids
See also
 In Spanish: Grupo saliente para niños
In Spanish: Grupo saliente para niños
 | Stephanie Wilson |
 | Charles Bolden |
 | Ronald McNair |
 | Frederick D. Gregory |


